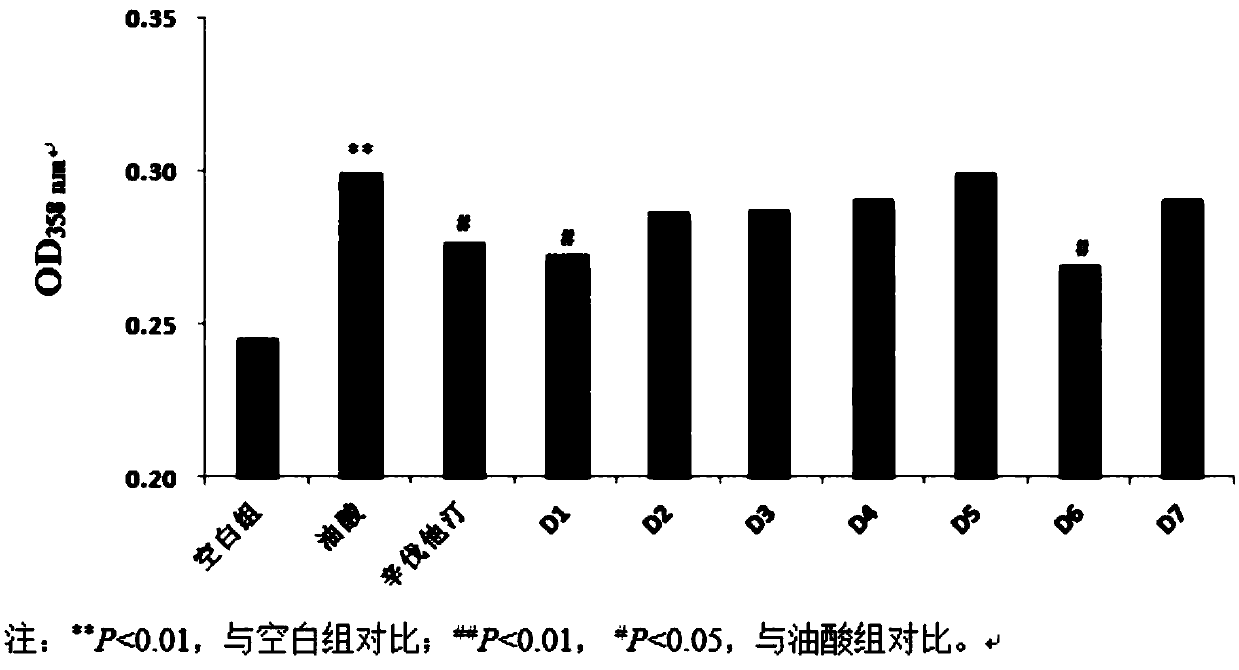
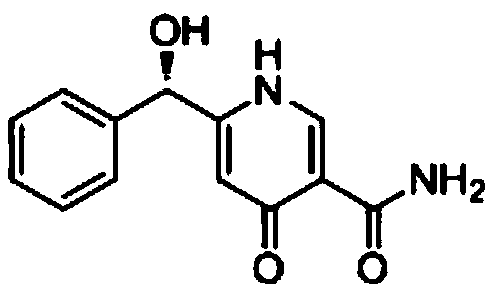
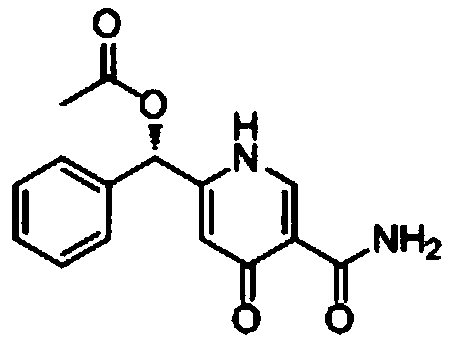
Synthetic method of pyridine alkaloid compounds and applications of these compounds
A pyridine alkaloid and synthesis method technology, which is applied in the field of synthesis of pyridine alkaloid compounds, can solve problems such as difficult structure-activity research, low content of PenipyridoneB, etc., and achieve significant lipid-lowering activity and good development and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1: the chemical synthesis of compound B
[0013]
[0014] A (293.1mg, 1.2mmol), condensing agent EDCI (276.1mg, 1.44mmol), catalyst DMAP (29.3mg, 0.24mmol) were sequentially added to the reaction flask, vacuum decompression, nitrogen protection, 10ml of anhydrous dichloromethane was added, Stir at 0°C, then add glacial acetic acid (137ul, 2.4mmol), keep the reaction at low temperature for 1 hour, no material A is detected by thin-layer chromatography, transfer to room temperature, add a small amount of dilute hydrochloric acid, extract and dry, and concentrate under reduced pressure Afterwards, the target product B was obtained as a yellow oil by column chromatography (eluent: dichloromethane:methanol=30:1). Yield 78%.
[0015] Product B is analyzed and the results are as follows: 1 H NMR (500MHz, DMSO) δ12.43(s,1H),9.40(s,1H),8.37(s,1H),7.41(ddd,J=20.4,16.5,7.0Hz,5H),6.64(s, 1H), 6.39(s, 1H), 2.18(s, 3H)ppm. 13 C NMR(125MHz,DMSO)δ177.74,169.76,165.51,...
Embodiment 2
[0016] Embodiment 2: the chemical synthesis of compound D1
[0017]
[0018] Add B (200mg, 0.7mmol) and potassium carbonate (193.5mg, 1.4mmol) to the pressure tube in turn, reduce the pressure under vacuum, protect with nitrogen, add 5ml of anhydrous acetone, then add C1 (248ul, 3.5mmol), at 75 degrees Stir under the conditions for 36 hours, no material B was detected by thin-layer chromatography, cooled to room temperature, extracted and dried, concentrated under vacuum and reduced pressure, and the target product was obtained by column chromatography (eluent: ethyl acetate:petroleum ether=10:1) D1, white solid. Yield 52%.
[0019] Product D1 is analyzed, and the results are as follows: 1 H NMR (500MHz, DMSO) δ8.71(s, 1H), 7.72(s, 1H), 7.60(s, 1H), 7.46(d, J=7.3Hz, 2H), 7.32(tt, J=14.6, 7.2Hz, 4H), 6.66 (s, 1H), 5.16(t, J=5.3Hz, 1H), 4.28(t, J=4.3Hz, 2H), 3.77(dd, J=9.2, 4.9 Hz, 2H) ,2.18(s,3H)ppm. 13 CNMR (125MHz, DMSO) δ170.06, 165.09, 163.67, 163.61, 152.03, 139.01...
Embodiment 3
[0020] Embodiment 3: the chemical synthesis of compound D2
[0021]
[0022] Add B (200mg, 0.7mmol) and potassium carbonate (193.5mg, 1.4mmol) to the pressure tube in turn, reduce the pressure under vacuum, protect with nitrogen, add 5ml of anhydrous acetone, then add C2 (181.7ul, 2.1mmol), at 75 Stir under the condition of 12 hours, thin-layer chromatography monitors that there is no raw material B, cool to room temperature, extract and dry, concentrate under vacuum and reduce pressure, and obtain the target product by column chromatography (eluent is dichloromethane:methanol=50:1) D2, white solid. Yield 33%.
[0023] Product D2 is analyzed, and the results are as follows: 1 H NMR (500MHz,DMSO)δ8.60(s,1H),7.66(s,1H),7.57(s,1H),7.47–7.43(m,2H),7.37–7.32(m,2H),7.32– 7.28(m,1H), 7.26(s,1H),6.65(s,1H),6.06(ddt,J=17.2,10.6,5.4Hz,1H),5.45(dd,J=17.3,1.6Hz,1H) ,5.32(dd,J=10.6,1.4Hz,1H),4.84(d,J=5.3Hz,2H),2.18 (s,3H)ppm. 13 C NMR(125MHz,DMSO)δ169.57,165.03,162.70,162.38,150.74...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com