A kind of bursa active pentapeptide and application of promoting the immune response of aiv and/or ndv vaccine
A bursal polypeptide and bursa technology, applied in medical preparations containing active ingredients, peptides, antibody medical ingredients, etc., can solve the problem of consuming a lot of manpower and material resources, can not effectively protect the attack, and strengthen the stress response of chickens and other problems, to achieve the effect of improving antigen presentation response, promoting extensive immune enhancement, and improving vaccine immune efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
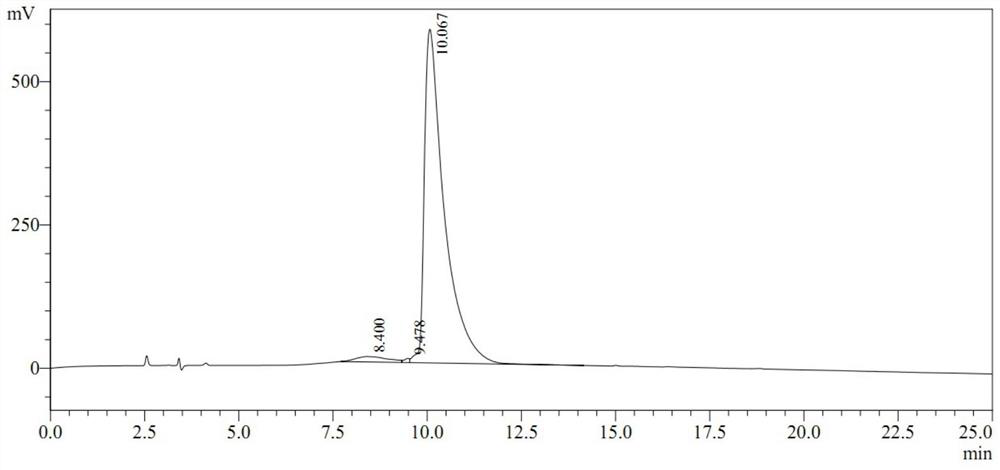
[0034] 1. Isolation and identification of pentapeptide
[0035] Wash 50 g of AA broiler bursa tissues without fascia and adipose tissue once with 0.85% physiological saline (precooled to 4-10° C.). After draining, place the bursa tissue in a tissue masher, add pre-cooled saline, and homogenize at high speed three times, 30 seconds each time, and keep the homogenization process at 0-10°C. Then, the homogenate was ultrasonically lysed twice at 4°C, 5 min each time. Then the lysate was heated to 80°C, kept warm for 5min, then quickly placed on ice, and cooled to 10°C. The lysate was then refrigerated and centrifuged for 30 minutes (4000×g / min). The supernatant was collected and freeze-thawed twice. Then the supernatant was centrifuged at high speed at 12000g / min, 4°C, 30min. The supernatant was collected for ultrafiltration, and the ultrafiltrate with a molecular weight below 1000 Da was collected, that is, the crude extract of the bursa tissue. After freeze-drying, dilute w...
Embodiment 2
[0037] 1. Artificially synthesized pentapeptide
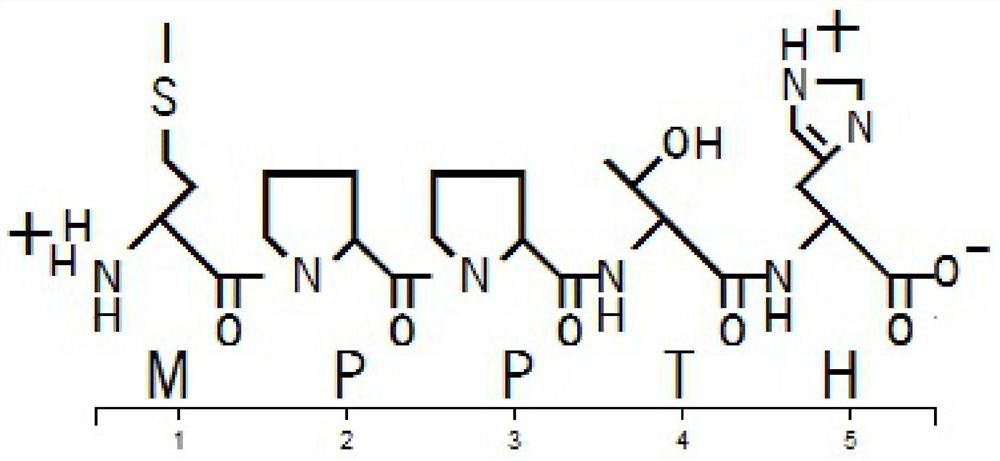
[0038] A commercial peptide synthesis company was entrusted to synthesize the peptide according to the MPPTH sequence (SEQ ID No.1), with a purity of 99.936%.
[0039] 2. Vaccines
[0040] Avian influenza and Newcastle disease dual inactivated vaccine (purchased from Nanjing Tianbang Biotechnology Co., Ltd.).
[0041] 3. Mouse immunization experiment
[0042] 3.1 Grouping of experimental animals and mice
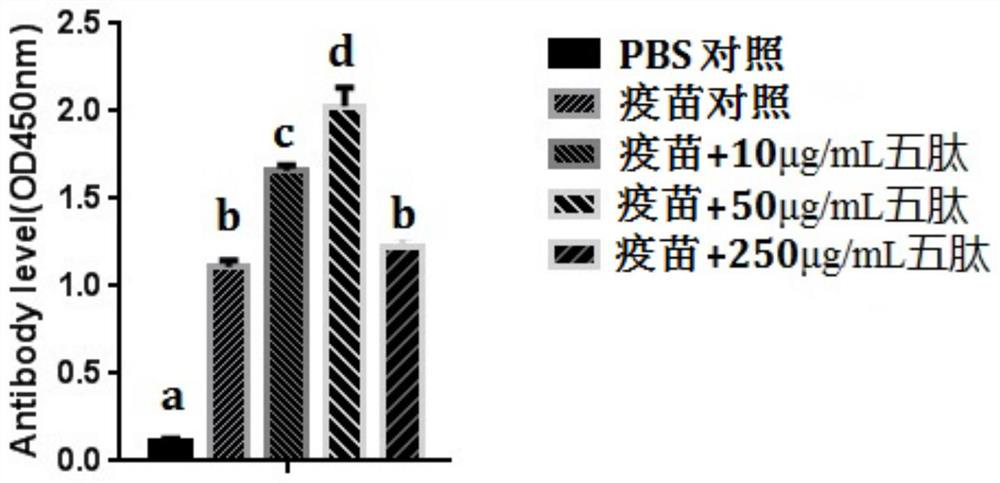
[0043] The BALB / C mice were randomly divided into five groups, 10 in each group: (I) PBS control group (each immunized with 0.2ml PBS); (II) AIV+NDV double inactivated vaccine immunization group (each immunized with inactivated vaccine) live vaccine 0.2ml); (III~V) AIV+NDV double inactivated vaccine+pentapeptide group (pentapeptide concentrations were 10, 50, 250 μg / mL); intraperitoneal injection was used to treat the corresponding groups of mice respectively Immunized twice. The immunization interval was two weeks, 0.2m...
Embodiment 3
[0051] 1. Artificially synthesized pentapeptide
[0052] A commercial peptide synthesis company was entrusted to synthesize the peptide according to the MPPTH sequence (SEQ ID No.1), with a purity of 99.936%.
[0053] 2. Vaccines
[0054] Avian influenza and Newcastle disease dual inactivated vaccine (purchased from Nanjing Tianbang Biotechnology Co., Ltd.).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com