A hydrate kinetic inhibitor
A kinetic inhibitor, hydrate technology, applied in drilling compositions, chemical instruments and methods, etc., can solve the problems of unsuitable high supercooling degree, low cloud point, not widely used, etc., and reduce the cost of reagents , cost reduction, good inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of hydroxyl-modified polyvinyl caprolactam (PVCap-OH) comprises the following steps:
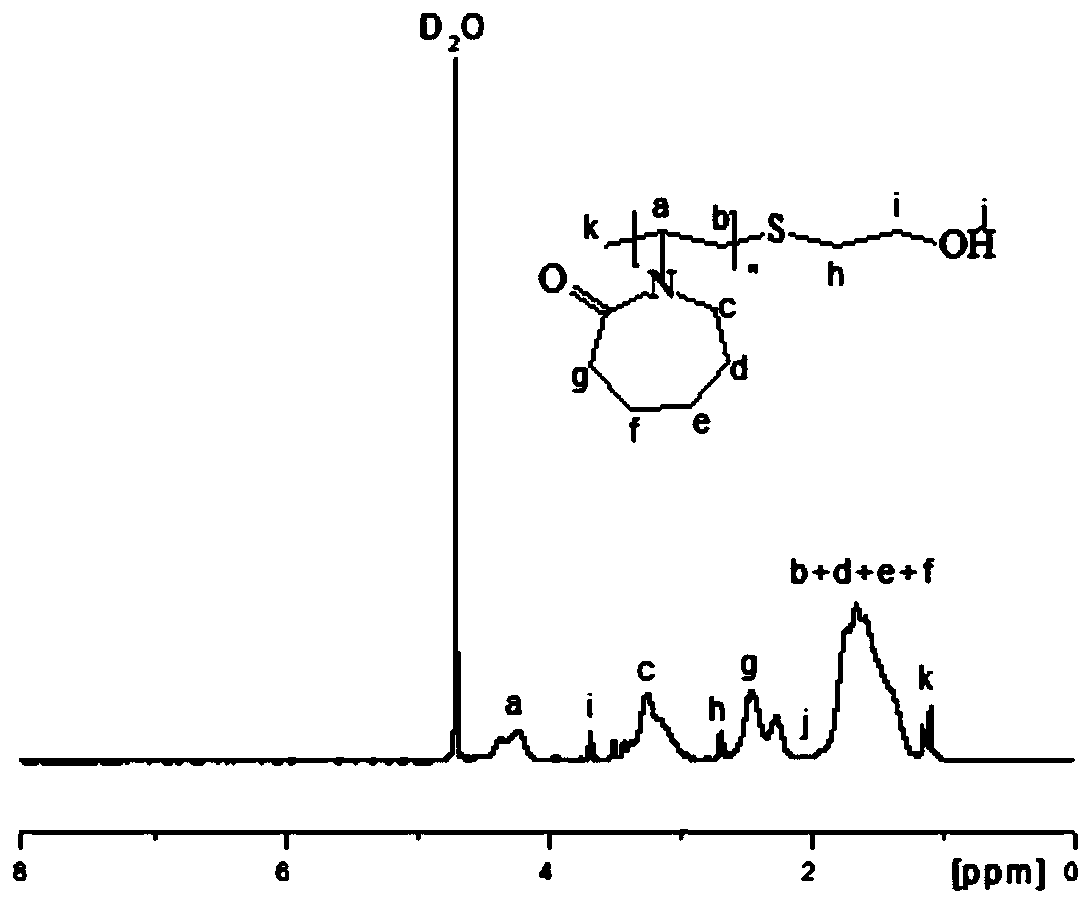
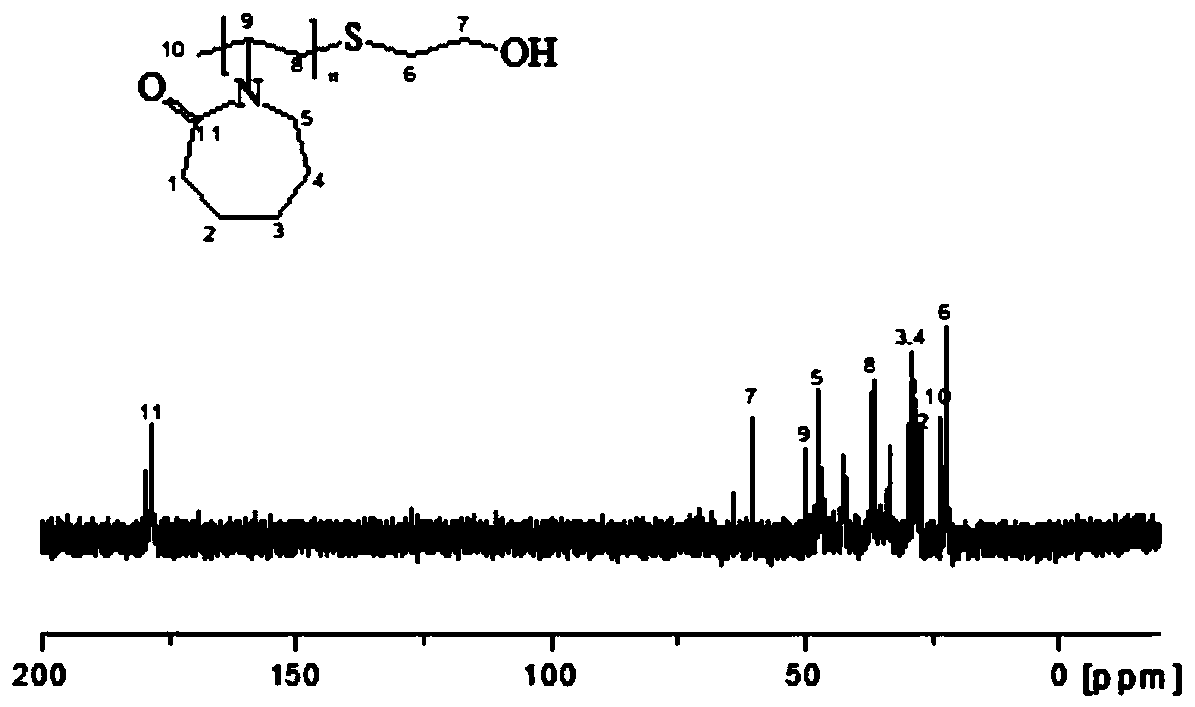
[0029] (1) Weigh 118mg (0.72mmol) of azobisisobutyronitrile, 10g (72mmol) of N-vinylcaprolactam, 280mg (3.6mmol) of mercaptoethanol, and 150mL of isopropanol in a 250mL three-necked flask, and stir until dissolved. After sealing with a rubber stopper, vacuum-purge nitrogen for 3 times; react at 85°C for 24 hours in a nitrogen atmosphere, dry the reaction solution by rotary evaporation at 50°C, and cool to room temperature to obtain a crude product, which is dissolved in tetrahydrofuran , and then precipitated with n-hexane, the solid-to-liquid ratio of the crude product to THF is 1:3g / mL, the volume of n-hexane is 5 times the volume of THF, the resulting precipitate is washed with a large amount of anhydrous ether, filtered, and dried to obtain hydrate Kinetic inhibitor PVCap-OH.
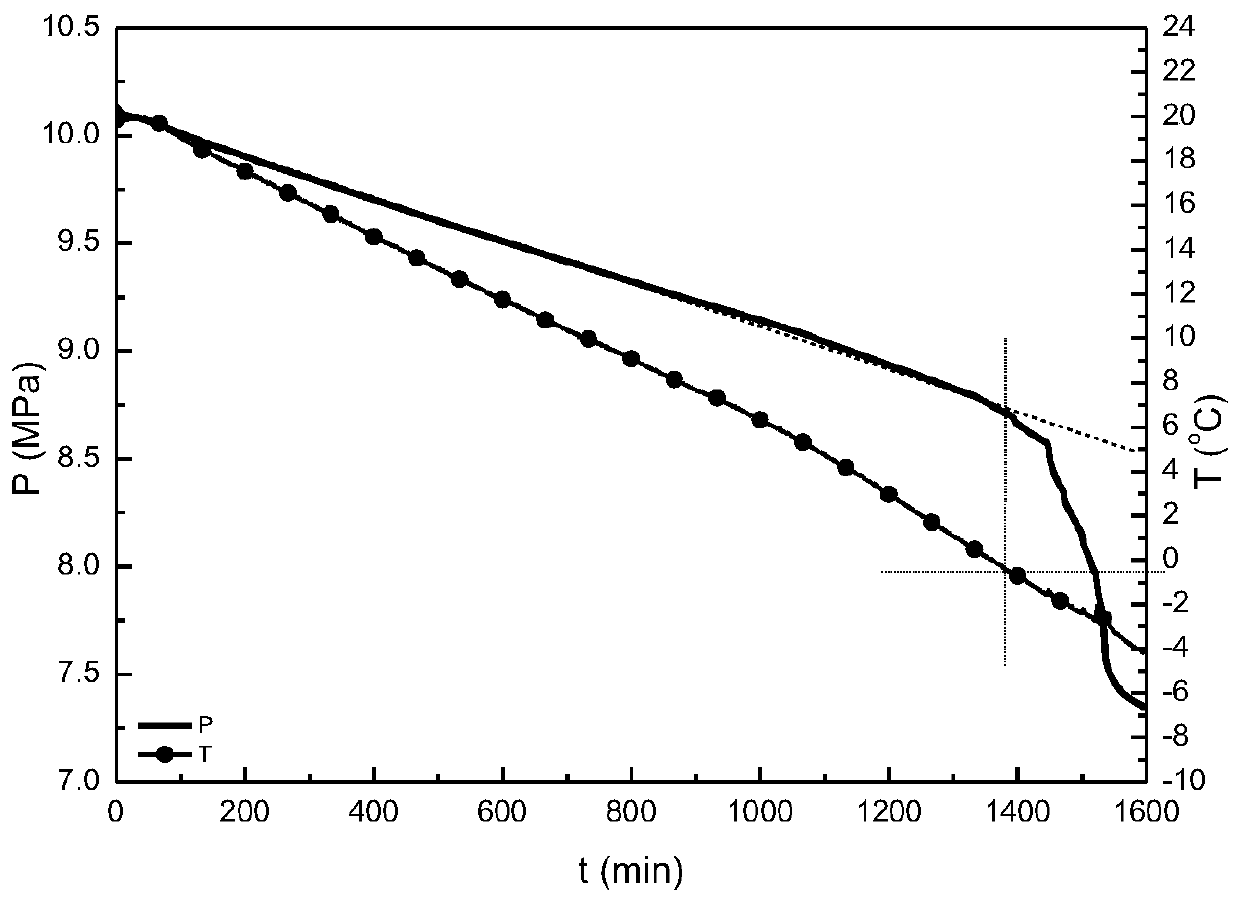
[0030] The obtained hydrate kinetics inhibitor PVCap-OH was detected by C-NMR and H-spe...
Embodiment 2
[0036] The preparation of hydroxyl-modified polyvinyl caprolactam (PVCap-OH) comprises the following steps:
[0037] (1) Weigh 0.36mmol of azobisisobutyronitrile, 72mmol of N-vinylcaprolactam, 0.72mmol of mercaptoethanol, and 10mL of isopropanol in a 250mL three-neck flask, stir until dissolved, seal with a rubber stopper, and then vacuum-vent Nitrogen 3 times; react at 30°C for 40 hours in a nitrogen atmosphere, dry the reaction solution by rotary evaporation at 30°C, cool to room temperature to obtain a crude product, dissolve the obtained crude product in tetrahydrofuran, and then precipitate it with n-hexane, the crude product The solid-liquid ratio with THF is 1:1g / mL, the volume of n-hexane is twice the volume of THF, the obtained precipitate is washed with a large amount of anhydrous ether, filtered, and dried to obtain the hydrate kinetic inhibitor PVCap-OH.
Embodiment 3
[0039] The preparation of hydroxyl-modified polyvinyl caprolactam (PVCap-OH) comprises the following steps:
[0040] (1) Weigh 0.36mmol of azobisisobutyronitrile, 72mmol of N-vinylcaprolactam, 7.2mmol of mercaptoethanol, and 200mL of isopropanol in a 250mL three-necked flask, stir until dissolved, seal with a rubber stopper, and then vacuum-vent Nitrogen 3 times; react at 90°C for 6 hours in a nitrogen atmosphere, dry the reaction solution by rotary evaporation at 85°C, cool to room temperature to obtain a crude product, dissolve the obtained crude product in tetrahydrofuran, and then precipitate it with n-hexane, the crude product The solid-liquid ratio with THF is 1:5g / mL, and the volume of n-hexane is 10 times the volume of THF. The resulting precipitate is washed with a large amount of anhydrous ether, filtered, and dried to obtain the hydrate kinetic inhibitor PVCap-OH.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com