Chiral polyester compounds and preparation method thereof
A compound and polyester technology, applied in biochemical equipment and methods, methods based on microorganisms, microorganisms, etc., to achieve the effect of single configuration and broad application development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
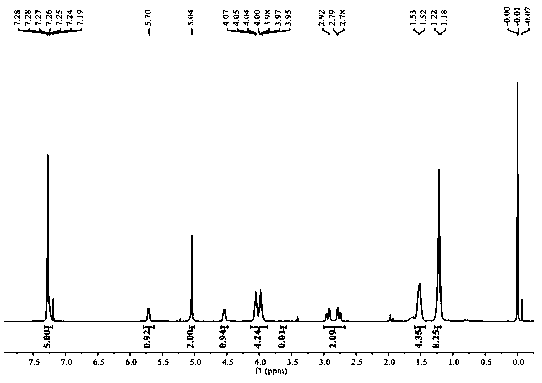
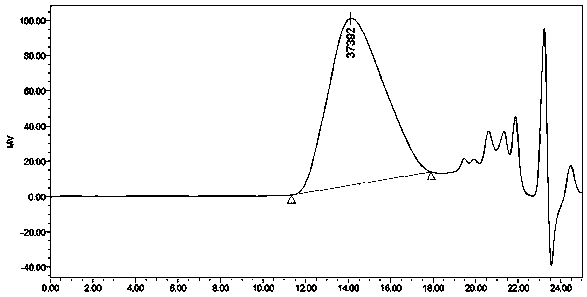
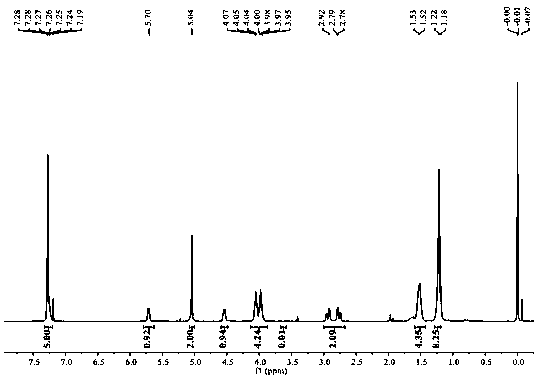
Image
Examples
Embodiment 1
[0033] Prepare a chiral polyester compound:
[0034]
[0035] 1) Weigh 1.0mmol N-benzyloxycarbonyl-L-aspartic acid dimethyl ester, 1.1mmol 1,4-butanediol and dissolve in 1.0mL toluene, add Candida antarctica lipase B (enzyme The amount used is 15% of the mass of N-benzyloxycarbonyl-L-aspartic acid dimethyl ester), pre-polymerized at normal pressure and temperature at 50°C for 24 hours, then kept the reaction temperature constant at an absolute pressure of 0.01 Polymerization reaction 48h under the condition of MPa, collect reaction product;
[0036] 2) The reaction product obtained in step 1) was dissolved in 1.0 mL of dichloromethane solution, filtered, and the filtrate was distilled under reduced pressure until viscous to obtain a polymer;
[0037] 3) The polymer obtained in step 2) was dissolved in 1.0 mL of a mixed solution of methanol and dichloromethane with a volume ratio of 5:1, and recrystallized, the macromolecular polymer was precipitated, filtered, and the filt...
Embodiment 2
[0041] Prepare a chiral polyester compound:
[0042]
[0043] 1) Weigh 1.0mmol N-benzyloxycarbonyl-L-aspartic acid dimethyl ester, 1.1mmol 1,8-octanediol and dissolve in 1.0mL toluene, add Candida antarctica lipase B (enzyme catalyst The amount used is 25% of the mass of N-benzyloxycarbonyl-L-aspartic acid dimethyl ester), pre-polymerized at normal pressure and temperature at 80°C for 24 hours, then kept the temperature constant at an absolute pressure of 0.01MPa Polymerization reaction 24h under the condition of condition, collect reaction product;
[0044] 2) The reaction product obtained in step 1) was dissolved in 1.0 mL of dichloromethane solution, filtered, and the filtrate was distilled under reduced pressure until viscous to obtain a polymer;
[0045] 3) The polymer obtained in step 2) was dissolved in 1.0 mL of a mixed solution of methanol and dichloromethane with a volume ratio of 5:1, recrystallized, and a macromolecular polymer was precipitated, filtered to obt...
Embodiment 3
[0053] Prepare a chiral polyester compound:
[0054]
[0055] 1) Weigh 1.0mmol of N-benzyl-L-aspartic acid dimethyl ester, 1.1mmol of 1,12-dodecanediol and dissolve it in 1.0mL of toluene, add Candida antarctica lipase B (enzyme catalyst The amount used is N-benzyl-L-aspartic acid dimethyl ester mass 30%), under the condition of normal pressure and temperature of 80 ℃ pre-polymerization reaction for 24h, then keep the temperature constant, under the condition of absolute pressure 0.01MPa Polymerization reaction 48h, collect reaction product;
[0056] 2) The reaction product obtained in step 1) was dissolved in 1.0 mL of dichloromethane solution, filtered, and the filtrate was distilled under reduced pressure until viscous to obtain a polymer;
[0057] 3) The polymer obtained in step 2) was dissolved in 1.0 mL of a mixed solution of methanol and dichloromethane with a volume ratio of 5:1, recrystallized, and a macromolecular polymer was precipitated, filtered to obtain 180 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com