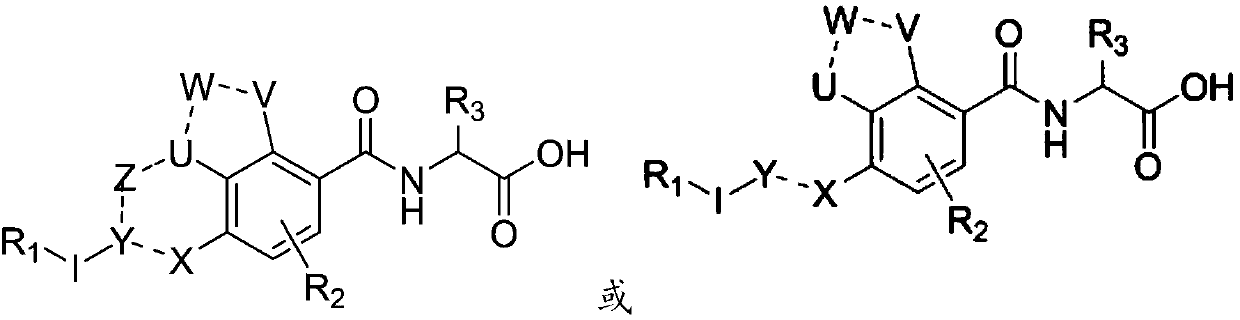
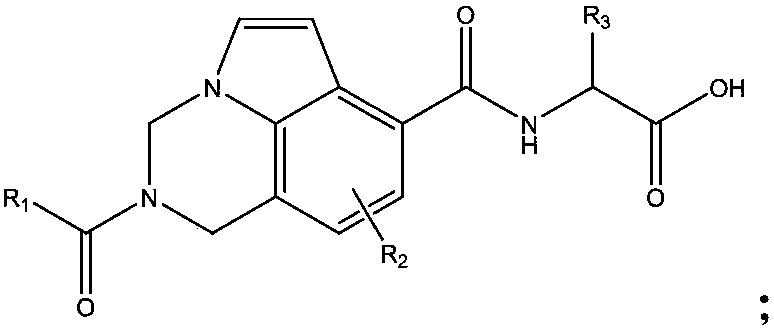
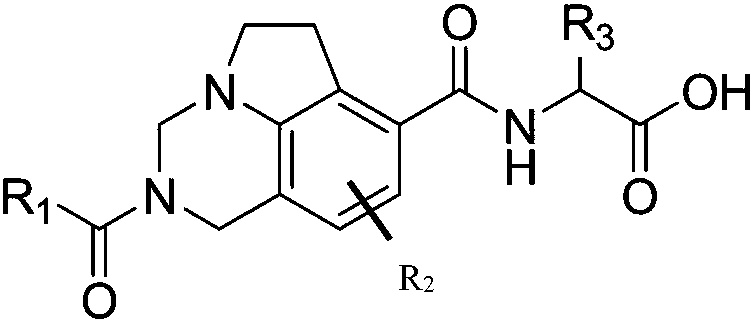
Inhibitor for immune cell migration
An immune cell and inhibitor technology, which can be used in anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc., and can solve problems such as skin side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117]
[0118] (S)-2-(8-Chloro-2-(benzofuran-6-carbonyl)-2,3-dihydro-1H-pyrrole[3,2,1-ij]quinazoline-7-carboxamide base)-3-(3-(methylsulfonyl)phenyl)propionic acid
[0119] The specific reaction equation is as follows:
[0120]
[0121] Step A: Dimethyl 2-amino-5-chloroterephthalate (Compound 1-1)
[0122]To a vigorously stirred solution of dimethyl 2-aminoterephthalate (10 g, 48 mmol) in isopropanol (1.5 L) was added NCS (7.34 g, 55 mmol) over a period of 5 minutes at room temperature. After the addition was complete, the reaction mixture was heated to reflux for 48 hours. After cooling to room temperature, the solvent was concentrated and the residue was purified by flash chromatography eluting with 5% ethyl acetate in petroleum jelly to give the desired product (6.4 g, 55%) as a pale yellow crystalline solid. LCMS ESI (+) m / z: 244 (M+1). 1 H NMR (600MHz, CDCl3) δ7.92(s,1H),7.10(s,1H),5.71(brs,2H),3.92(s,3H),3.90(s,3H).
[0123] Step B: Dimethyl 2-amino-3-bromo-5...
Embodiment 2
[0150]
[0151] (S)-2-(8-Chloro-2-(1H-indole-6-carbonyl)-2,3-dihydro-1H-pyrrole[3,2,1-ij]quinazoline-7- Formamido)-3-(3-(methylsulfonyl)phenyl)propanoic acid
[0152] The title compound was prepared according to the procedure of Example 1, Steps M and N, using 1H-indole-6-carboxylic acid instead of compound benzofuran-6-carboxylic acid to give a white solid. LCMS ESI (+) m / z: 605 (M+1). 1 H NMR(600MHz,DMSO)δ12.89(brs,1H),11.39(s,1H),8.78(d,J=8.2Hz,1H),7.89(s,1H),7.79(d,J=7.8Hz ,1H),7.70(d,J=7.5Hz,1H),7.63(d,J=8.1Hz,1H),7.59(t,J=7.7Hz,1H),7.52(s,1H),7.53–7.46 (m,1H),7.46(s,1H),7.04(d,J=8.2Hz,1H),7.03–6.93(m,1H),6.52(s,1H),6.13(s,1H),5.99– 5.78(m,2H),5.14–4.95(m,2H),4.85–4.69(m,1H),3.30–3.26(m,1H),3.12(s,3H),3.10–3.03(m,1H).
Embodiment 3
[0154]
[0155] (S)-2-(8-chloro-2-(pyrazol[1,5-a]pyridine-2-carbonyl)-2,3-dihydro-1H-pyrrole[3,2,1-ij] Quinazoline-7-carboxamido)-3-(3-(methylsulfonyl)phenyl)propionic acid
[0156] The title compound was prepared according to the method of Steps M and N of Example 1, using pyrazol[1,5-a]pyridine-2-carboxylic acid instead of compound benzofuran-6-carboxylic acid to obtain a white solid. LCMS ESI (+) m / z: 606 (M+1). 1 H NMR (600MHz, DMSO) δ12.89(brs, 1H), 8.76(d, J=8.0Hz, 1H), 8.75(d, J=6.5Hz, 1H), 7.88(s, 1H), 7.79(d ,J=8.3Hz,1H),7.77(d,J=10.1Hz,1H),7.69(d,J=7.6Hz,1H),7.58(t,J=7.7Hz,1H),7.63–7.40(m ,1H),7.33(t,J=7.7Hz,1H),7.10–7.01(m,1H),7.15–6.92(m,1H),6.90(s,1H),6.30–6.18(m,1H), 6.17–6.06(m,1H),,6.06–5.93(m,1H),5.44–5.31(m,1H),5.23–5.08(m,1H),4.82–4.74(m,1H),3.32(dd, J=14.3,4.3Hz,1H),3.11(s,3H),3.05(dd,J=13.5,11.4Hz,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com