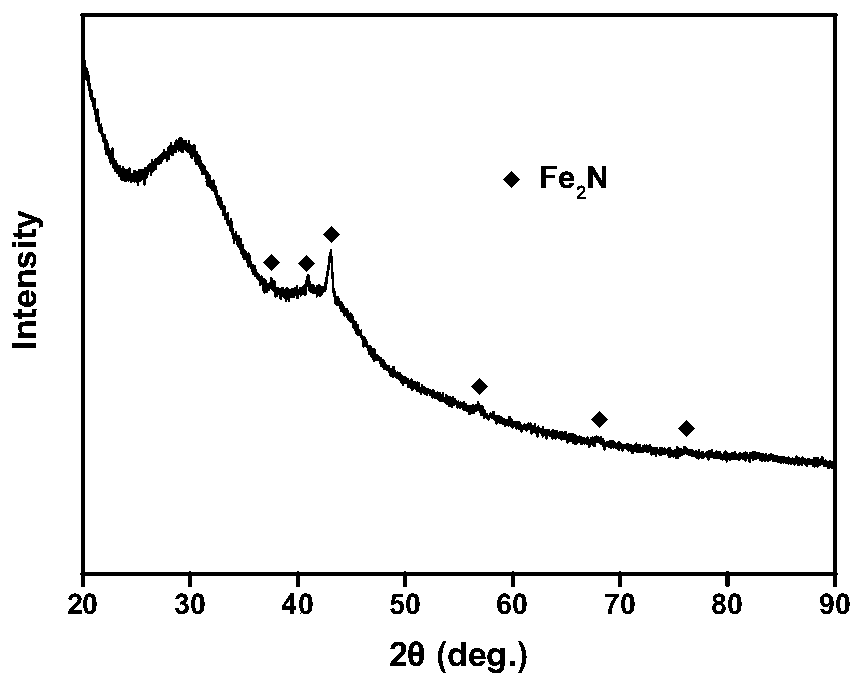
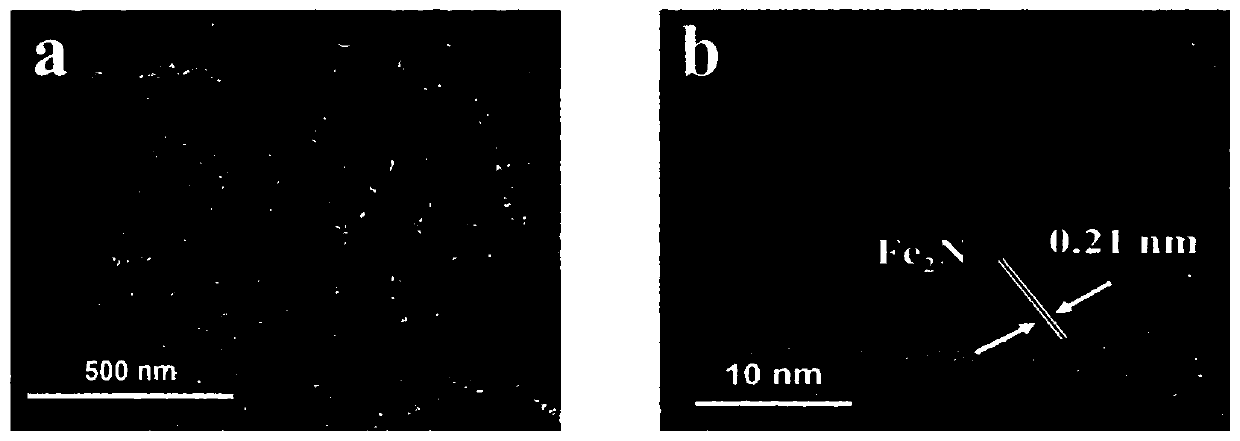
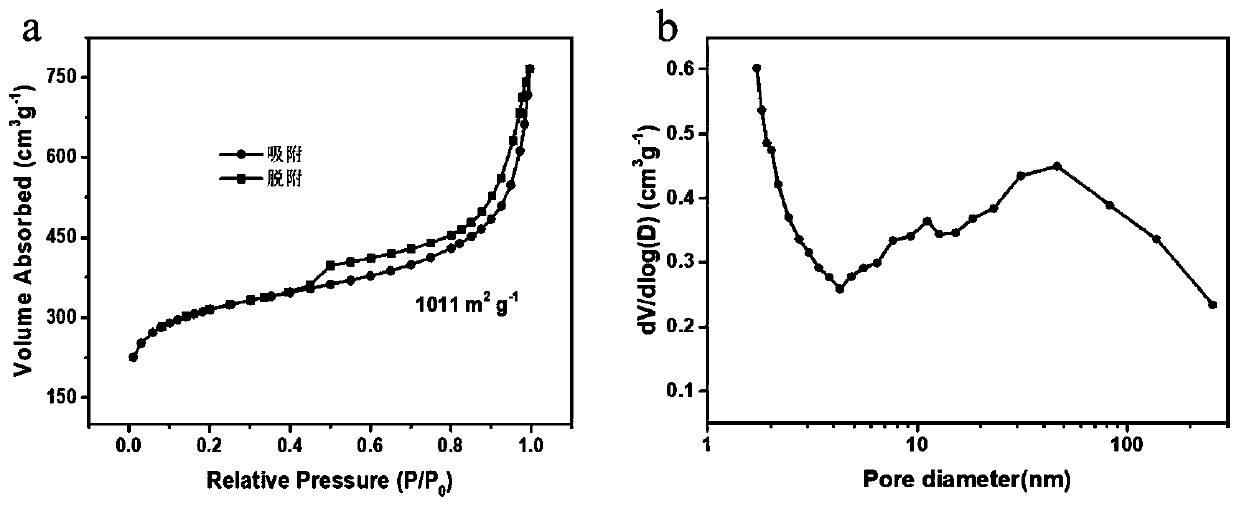
ZIFs-derived non-noble metal oxygen reduction catalyst and preparation method thereof and application thereof
A non-precious metal and catalyst technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of low utilization rate of active sites, low density of active centers, poor activity and durability, etc., and achieve good methanol resistance performance, material Low cost, effect of improving oxygen reduction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] (1) Dissolve 3.2g of 2-methylimidazole in 50mL of methanol, and another 1.86g of Zn(NO 3 ) 2 ·6H 2 O and 0.043g FeSO 4 ·7H 2 O (the ratio of the amount of iron to zinc is 1:40, the ratio of the amount of zinc to 2-methylimidazole is 1:6.23) is dissolved in 15mL of methanol, and the above solution is added, and the mixed solution is placed under ultrasonic conditions. The Fe-doped ZIFs precursor was obtained by reacting for 2 h, and the precursor was dried at 60 °C for 3 h.
[0071] (2) The dried precursor was heat-treated in a vacuum tube furnace at 950 °C for 2 h under the protection of a nitrogen atmosphere, with a heating rate of 5 °C / min, and then naturally dropped to room temperature.
[0072] (3) After the sample is lowered to room temperature, it is immersed in 0.5M H 2 SO 4 The solution was acid-washed at 80°C for 8h, then filtered, fully washed with deionized water, and then dried at 60°C for 3h.
[0073] (4) The dried sample was heated to 850°C at a rat...
Embodiment 2
[0086] Prepare ZIFs derivative non-noble metal oxygen reduction catalyst substantially according to the same method as Example 1, the difference is: in step (1) FeSO 4 ·7H 2 The feeding amount of O is changed into 0.058g (the ratio of the amount of substance of iron and zinc nitrate is 1:30) by 0.043g, the catalyst obtained is in 0.1M HClO 4 The half-wave potential obtained by testing the oxygen reduction polarization curve in the solution is 0.791V.
Embodiment 3
[0088] Prepare ZIFs derivative non-noble metal oxygen reduction catalyst substantially according to the same method as Example 1, the difference is: in step (1) FeSO 4 ·7H 2 The charging capacity of O is changed into 0.035g (the ratio of the amount of substance of iron and zinc nitrate is 1:50) by 0.043g, the catalyst obtained is in 0.1M HClO 4 The half-wave potential obtained by testing the oxygen reduction polarization curve in the solution is 0.782V.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Mesopore diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com