Blood protection solution for preserving cfDNAs (cell-free DNAs) in whole blood at room temperature
A protective liquid and blood technology, which is applied in the field of blood protective liquid for cfDNA preservation in whole blood at room temperature, can solve the problems of low storage temperature, high economic cost, and short storage time, and achieve the goals of avoiding pollution, prolonging storage time, and preventing rupture Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]Example 1, Preparation of Blood Protection Solution for Preserving cfDNA in Whole Blood at Normal Temperature
[0043] The blood protection solution for cfDNA in whole blood consists of 10-100g / L 1,3-dimethylol-5,5-dimethylhydantoin, 0.1-1mL / L Proclin300, 10-100g / L EDTA salt , 1-10g / L ammonium sulfate, 1-10g / L ATA, glycerol and buffer of 1-10mL / L consist of tris-hydrochloric acid with a concentration of 10mM (pH value 8.0) solution.
[0044] The concentration is 10mM tris-hydrochloric acid solution, the solute is 10mM tris-salt (Tris), and the solvent is water.
[0045] The specific formula is as follows:
[0046] cfDNA blood protection solution formula 1: 50g / L 1,3-dimethylol-5,5-dimethylhydantoin, 0.2mL / L Proclin300, 20g / L EDTA-K2, 1g / L sulfuric acid Ammonium, 1g / L ATA, 5mL / L of glycerol and a buffer solution, the buffer solution is a tris-hydrochloric acid solution with a concentration of 10mM (pH value 8.0);
[0047] cfDNA blood protection solution formula two: 5...
Embodiment 2
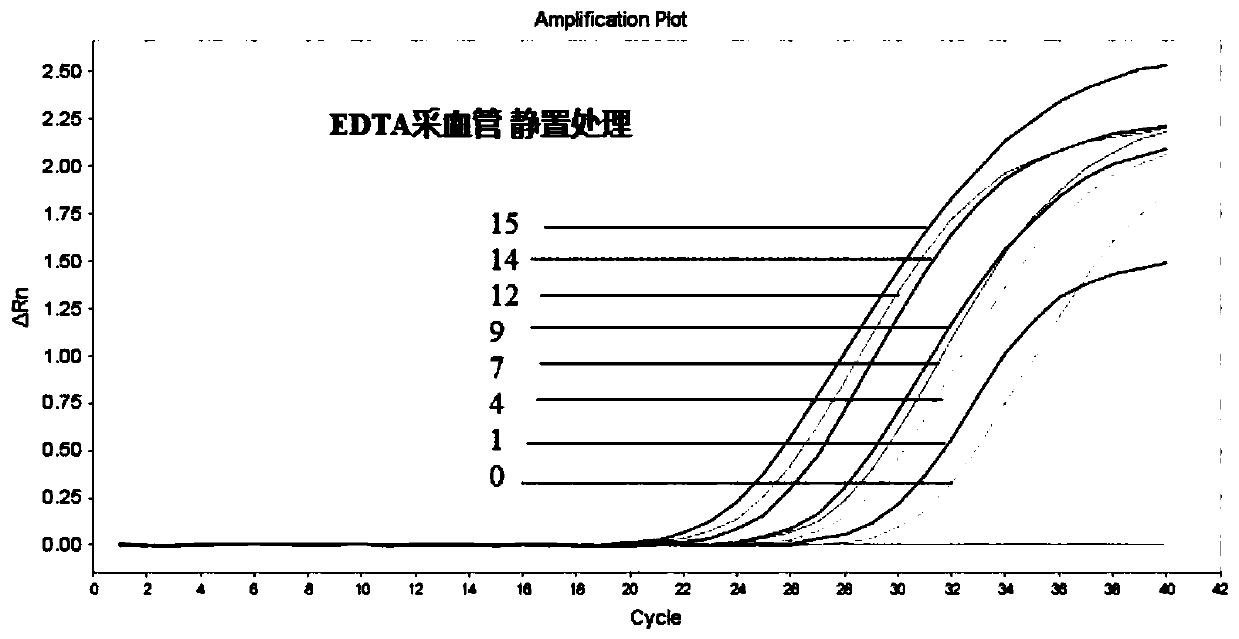
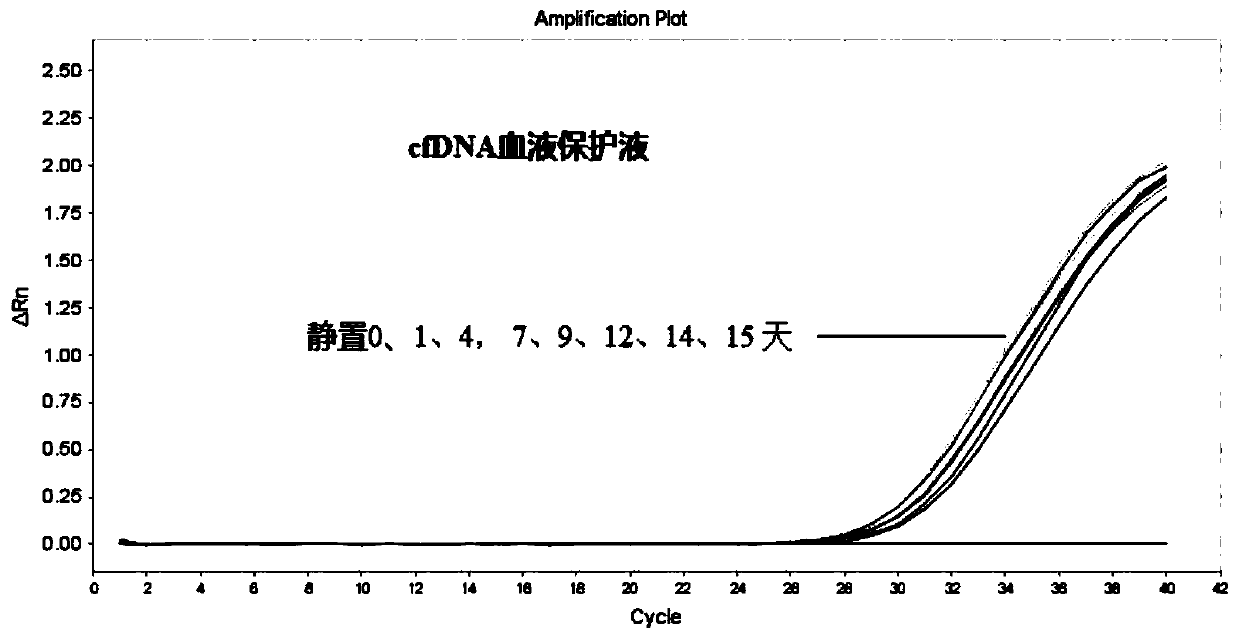
[0048] Embodiment 2, detection of blood protection solution room temperature stability
[0049] The blood sample and cfDNA blood protection solution were mixed thoroughly at a ratio of 5:1 to prevent coagulation due to insufficient contact.
[0050] Prepare anticoagulant according to formula one group in embodiment 1, and carry out comparative test with common EDTA blood collection tube, concrete steps are as follows:
[0051] 1. Use EDTA blood collection tubes and vacuum blood collection tubes added with the blood protection solution provided in Example 1 to collect the same amount of peripheral blood respectively. The total volume of the blood collection tubes is 5mL; the volume ratio of blood protection solution to peripheral blood is 1:5, vacuum Accuracy error ≤ 10%;
[0052] 2. The collected blood is placed at room temperature;
[0053] 3. Separate the plasma on days 0, 1, 4, 7, 9, 12, 14, and 15; the step of separating the plasma is to put the blood into a centrifuge t...
Embodiment 3
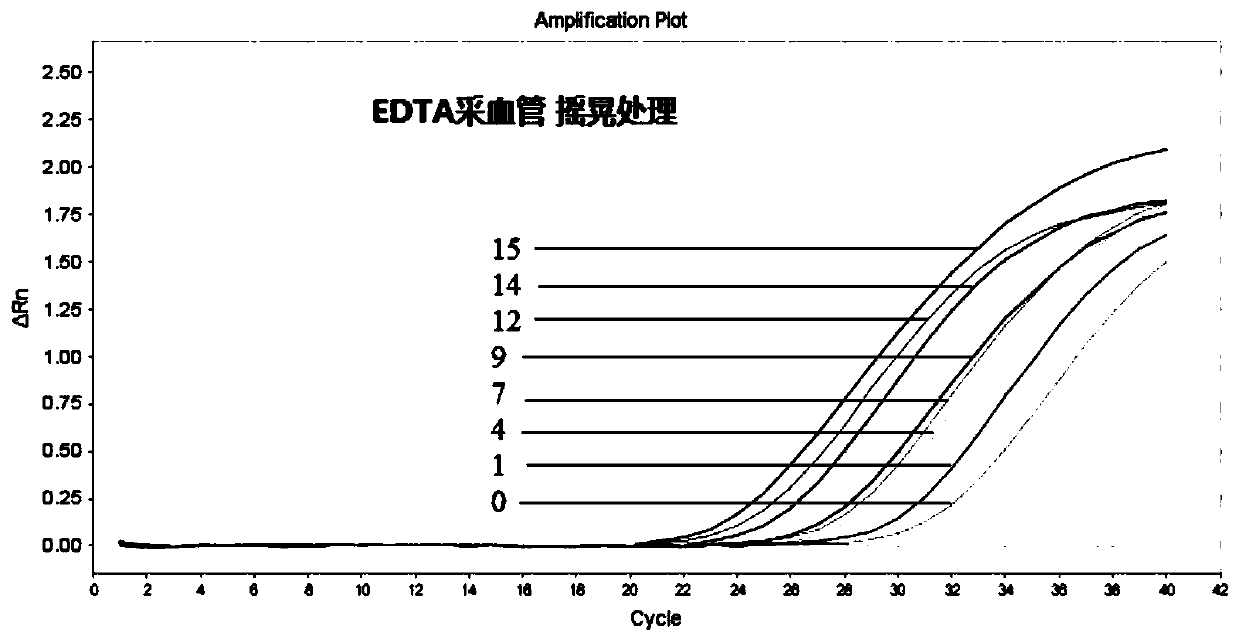
[0066] Embodiment 3, transport stability of blood protection solution
[0067] According to the vacuum blood collection tubes and common EDTA blood collection tubes prepared by formula 2 in Example 1, the same amount of peripheral blood was respectively taken to carry out the simulated transportation stability comparison test:
[0068] 1. The collected blood is placed at room temperature, and the blood is gently inverted and mixed twice a day to simulate the transportation environment, so as to observe the changes of blood cells and plasma DNA in the transportation environment.
[0069] 2. On days 0, 1, 4, 7, 9, 12, 14, and 15, separate the plasma in EDTA blood collection tubes and vacuum blood collection tubes added with blood protection solution;
[0070] 3. Extract the DNA in the separated plasma;
[0071] 4. Perform q-PCR verification of the housekeeping gene β-actin on the obtained DNA, and judge the test result according to the Ct value displayed by the fluorescent PCR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com