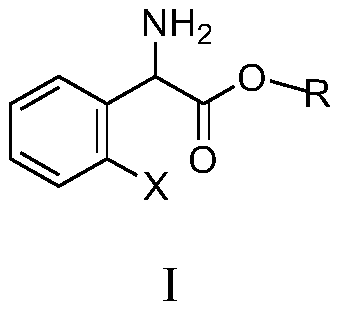
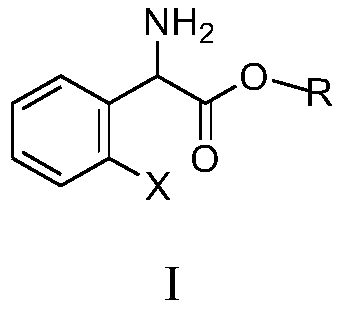
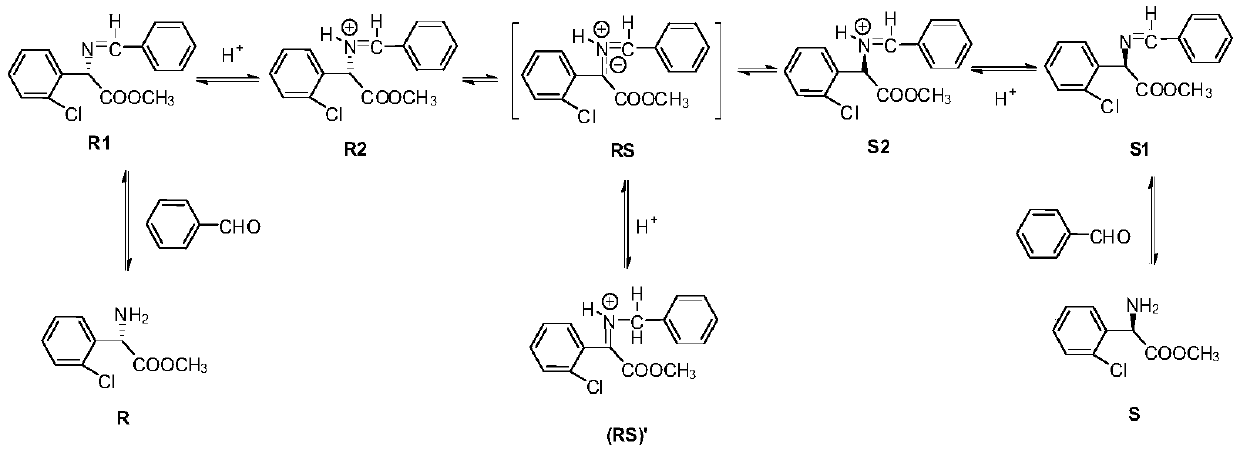
Substituted aromatic ring phenylglycine fatty alcohol ester resolution method
A technology of aromatic ring phenylglycine and fatty alcohol ester, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of complicated screening of solvents and resolving agents, large amount of solvent used, large loss of raw materials, etc., and achieves the saving of solvents and resolving agents. Use, the reaction time is short, the effect of improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Add L-(+)-tartaric acid (6.6g, 0.044mol) and anhydrous methanol (80ml) in the 100ml three-necked flask, stir at room temperature until completely dissolved, add 0.4g (0.003mol) acetophenone, and o-chlorobenzene The mixed solution of glycine methyl ester (8.0g, 0.04mol) and 16ml solvent (solvent is the mixture of acetone and methanol, the volume ratio of acetone and methanol is 1:5) is added dropwise in the reactor, and there is The solid precipitated, and it took about 4 hours (during which liquid chromatography, column chromatography or thin plate chromatography was launched to monitor the reaction, liquid chromatography, column chromatography or thin plate chromatography was used to judge the reaction progress by ΔRf value, and the ΔRf value was 1.38; the reaction process In liquid chromatography, column chromatography or thin layer chromatography development, capillary sampling 2 ~ 3mm, spot plate development measurement, R-(-)-o-chlorophenylglycine methyl ester tartr...
Embodiment 2
[0060] Add L-(+)-tartaric acid (7.0 g, 0.047 mol) and anhydrous methanol (96 ml) to a 100 ml three-necked flask, stir at room temperature until completely dissolved, add 0.3 g of acetophenone, and dissolve 2-fluorophenylglycine methyl ester (8.0g, 0.043mol) and 16ml solvent (solvent is the mixture of acetone and methanol, the volume ratio of acetone and methanol is 1: 6) the mixed solution is added dropwise in the reactor, there is solid to separate out in the process of dropping, It takes about 3 hours (during which liquid chromatography, column chromatography or thin-plate chromatography monitors the reaction, and liquid chromatography, column chromatography or thin-plate chromatography judges the reaction process by ΔRf value, ΔRf value is 1.85, and ΔRf is S-(+ )-2-fluorophenylglycine methyl ester tartrate and R-(-)-2-fluorophenylglycine methyl ester tartrate Rf difference, the developing agent is acetone and methanol, the ratio of the two is constantly adjusted in the exper...
Embodiment 3
[0062]Add L-(+)-tartaric acid (6.4 g, 0.042 mol) and anhydrous methanol (64 ml) into a 100 ml three-necked flask, and stir at room temperature until they are completely dissolved. Add 0.35g benzaldehyde, the mixed solution of 2-ethoxyphenylglycine methyl ester (8.0g, 0.038mol) and 16ml solvent (solvent is the mixture of acetone and methanol, the volume ratio of acetone and methanol is 1:4.5) Add dropwise in the reactor, solid is separated out in the process of dropping, takes about 3 hours (during liquid phase chromatography, column chromatography or thin plate chromatography monitor reaction, liquid phase chromatography, column chromatography or thin plate chromatography start with The ΔRf value judges the reaction process, and the ΔRf value is 1.52. ΔRf is the difference between S-(+)-2-ethoxyphenylglycine methyl ester tartrate and R-(-)-2-ethoxyphenylglycine methyl ester tartrate Rf Value, the developer is acetone and methanol, the ratio of the two is constantly adjusted in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com