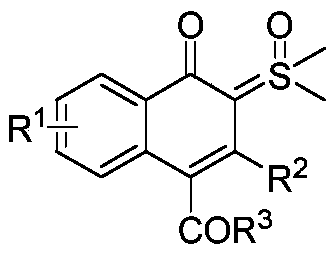
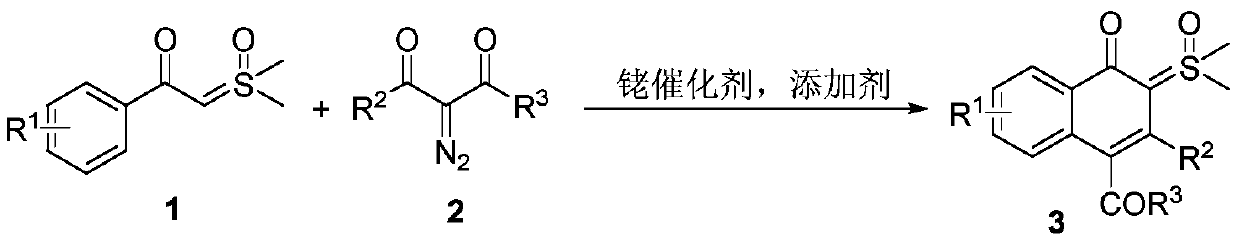
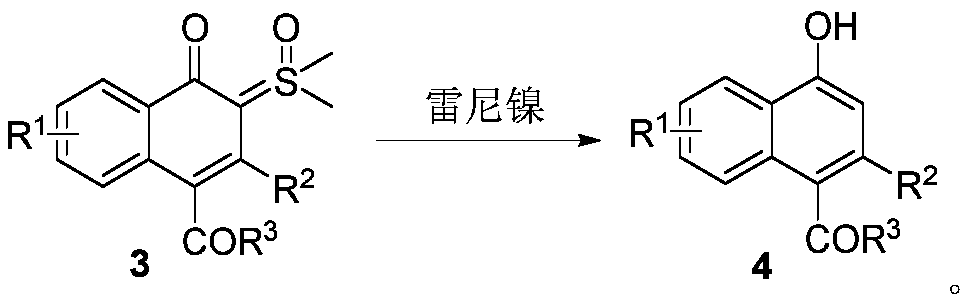
Synthesis and application of naphthalenone-sulfoxide ylide hybrid
A technology of sulfoxide leaf and naphthalene ketone is applied in the synthesis and application field of naphthalene ketone-sulfoxide ylide hybrid, and achieves the effects of wide application range, good application prospect and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] 1a (0.5mmol, 98mg), 2a (0.5mmol, 109mg), silver hexafluoroantimonate (27.5mg, 0.08mmol), [RhCp*Cl 2 ] 2 (12mg, 0.02mmol) and 2,2,2-trifluoroethanol (3mL), stirred at 80°C for 24h under nitrogen atmosphere. Then the reaction system was cooled to room temperature, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), the organic phases were combined, and washed with anhydrous Na 2 SO 4 After drying, the solvent was spin-dried and separated by silica gel column (ethyl acetate / methanol=20 / 1) to obtain the product 3a (79 mg, 43%) as a white solid. The characterization data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 )δ: 0.81(t, J=7.2Hz, 3H), 3.70(s, 6H), 3.84(q, J=7.2Hz, 2H), 7.24-7.28(m, 5H), 7.38(t, J=7.2 Hz,1H),7.53(t,J=7.6Hz,1H),7.63(dd,J 1 =8.4Hz,J 2 =0.8Hz,1H),8.32(d,J=8.0Hz,1H). 13C NMR (150MHz, CDCl 3 )δ: 13.7, 43.8, 60.9, 97.1, 118.0, 124.7, 124.9, 125.5, 127.2, 127.6, 129.3, 130.2, 131.1...
Embodiment 2
[0035] 1a (0.5mmol, 98mg), 2a (0.5mmol, 109mg), silver hexafluoroantimonate (27.5mg, 0.08mmol), [RhCp*Cl 2 ] 2 (12mg, 0.02mmol) and tetrahydrofuran (3mL), stirred at 80°C for 24h under nitrogen atmosphere. Then the reaction system was cooled to room temperature, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), the organic phases were combined, and washed with anhydrous Na 2 SO 4 After drying, the solvent was spin-dried and separated by silica gel column (ethyl acetate / methanol=20 / 1) to obtain the product 3a (20 mg, 11%) as a white solid.
Embodiment 3
[0037] 1a (0.5mmol, 98mg), 2a (0.5mmol, 109mg), silver hexafluoroantimonate (27.5mg, 0.08mmol), [RhCp*Cl 2 ] 2 (12mg, 0.02mmol) and acetonitrile (3mL), stirred at 80°C for 24h under nitrogen atmosphere. Then the reaction system was cooled to room temperature, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), the organic phases were combined, and washed with anhydrous Na 2 SO 4 After drying, the solvent was spin-dried and separated by silica gel column (ethyl acetate / methanol=20 / 1) to obtain the product 3a (28 mg, 15%) as a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com