Clathrate compound of camptothecin and acid-sensitive open-loop cucurbituril
An acid-sensitive, cucurbituril technology, which is applied in the field of targeted preparations of camptothecin drugs, can solve the problems of cyclodextrin inclusion without targeting and cumbersome derivatization process, and overcome low bioavailability and water solubility. The effect of poor performance and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: The inclusion compound of camptothecin and acid-sensitive split-loop cucurbituril contains camptothecin and acid-sensitive split-loop cucurbituril (a), and the structural formula of acid-sensitive split-loop cucurbituril is as follows:
[0028] , R 1 =CH 3 , R 2 =H or R 1 = H, R 2 = H;
[0029] Camptothecin and acid-sensitive secocucurbituril (a, R 1 =CH 3 , R 2 = H; R 1 = H, R 2 =H) Preparation of clathrates
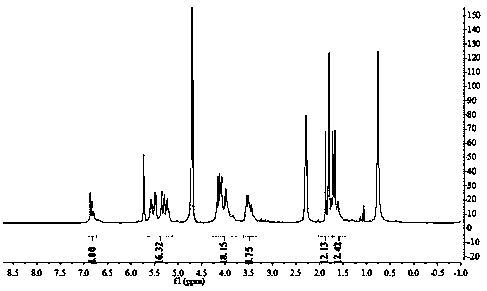
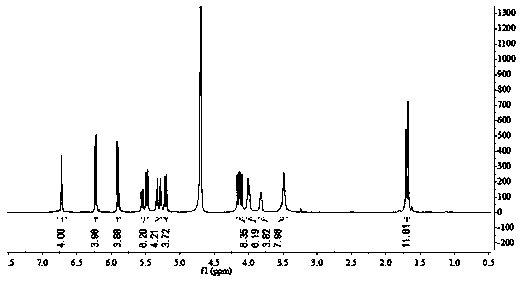
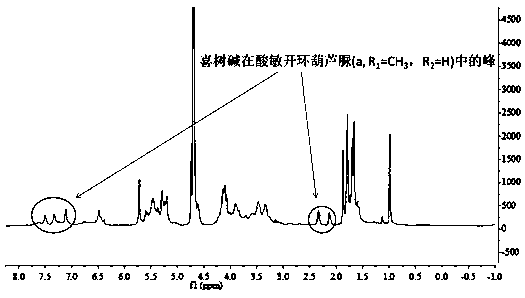
[0030] (1) 0.1 mmol acid-sensitive split-ring cucurbituril (a, R 1 =CH 3 , R 2 =H) into the ethanol solution, add 0.1mmol camptothecin, continue to stir in the dark for 24 hours, use a 4.5μm microporous membrane filter to remove insoluble matter, evaporate the solvent under reduced pressure, and obtain camptothecin and acid-sensitive split cucurbita Urea (a, R 1 =CH 3 , R 2 =H) powdery solid clathrate; from figure 1 , image 3 It can be seen that camptothecin and acid-sensitive secocucurbituril (a, R 1 =CH 3 , R 2 =H) The clathr...
Embodiment 2
[0037] Example 2: The inclusion compound of camptothecin and acid-sensitive split-loop cucurbituril contains camptothecin and acid-sensitive split-loop cucurbituril (b), wherein the structural formula of acid-sensitive split-loop cucurbituril is as follows:
[0038] ;
[0039] Camptothecin and acid-sensitive secocucurbituril (b, R 1 =CH 3 , R 2 = H; R 1 = H, R 2 =H) Preparation of clathrate
[0040] (1) 0.1 mmol acid-sensitive split-ring cucurbituril (b, R 1 =CH 3 , R 2 =H) into the ethanol solution, add 0.3mmol camptothecin, continue to stir in the dark for 48 hours, use a 4.5μm microporous membrane filter to remove insoluble matter, evaporate the solvent under reduced pressure, and obtain camptothecin and acid-sensitive split-ring cucurbit Urea (b, R 1 =CH 3 , R 2 =H) powdery solid clathrate;
[0041] (2) 0.1 mmol of acid-sensitive split-ring cucurbituril (b, R 1 = H, R 2 =H) into the ethanol solution, add 0.4mmol camptothecin, continue to stir in the dark fo...
Embodiment 3
[0047] Example 3: The inclusion compound of camptothecin and acid-sensitive split-loop cucurbituril contains camptothecin and acid-sensitive split-loop cucurbituril (c), wherein the structural formula of acid-sensitive split-loop cucurbituril is as follows:
[0048] ;
[0049] Camptothecin and acid-sensitive secocucurbituril (c, R 1 = H, R 2 =CH 3 ; 1 = H, R 2 =H) Preparation of clathrate
[0050] (1) 0.1 mmol acid-sensitive open-loop cucurbituril (R 1 = H, R 2 =CH 3 ) in ethanol solution, add 0.5mmol camptothecin, continue to avoid light and stir for 72h, use 4.5μm microporous membrane filter to remove insoluble matter, evaporate the solvent under reduced pressure, and obtain camptothecin drug and acid-sensitive split-ring cucurbituril (c, R 1 =CH 3 , R 2 =H) powdery solid clathrate;
[0051] (2) 0.1 mmol of acid-sensitive open-loop cucurbituril (c, R 1 = H, R 2 =H) into the ethanol solution, add 0.3mmol camptothecin, continue to stir in the dark for 84 hours,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com