A method for preparing 1,6-hexanediol by catalytic hydrogenolysis of 1,2,6-hexanetriol
A technology for catalyzing hydrogen and catalytic hydrogenolysis of hexanetriol, applied in chemical instruments and methods, reduction preparation of oxygen-containing functional groups, physical/chemical process catalysts, etc. Low-level problems, to achieve the effect of short reaction time, high yield and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Catalyst preparation and catalytic hydrogenolysis experiment
[0019] The catalyst is prepared by the impregnation method, the carrier is nano-zirconium dioxide, the aqueous solution of metal salt is impregnated in equal volume, and the impregnation is dried at 110°C for 12h, then roasted in 350°C air atmosphere for 3h, and finally reduced in 300°C hydrogen atmosphere for 2h. When activated carbon is the carrier, the air atmosphere is changed to high-purity nitrogen, and the other conditions are the same as before. Then take a certain amount of 1,2,6-hexanetriol, reaction solvent, catalyst and solid acid in a 25mL stainless steel reaction kettle, and pass hydrogen at a certain pressure to repeatedly replace the air in the kettle. Warm up to the specified temperature and react for a certain time. After the completion of the reaction, the reaction kettle was cooled to room temperature, and finally suction filtered and the reaction liquid was subjected to chromatographic ana...
Embodiment 2
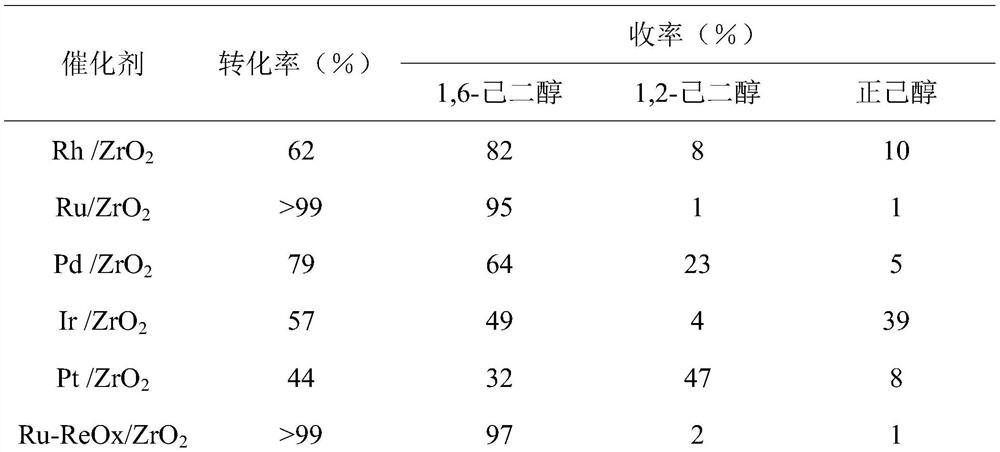
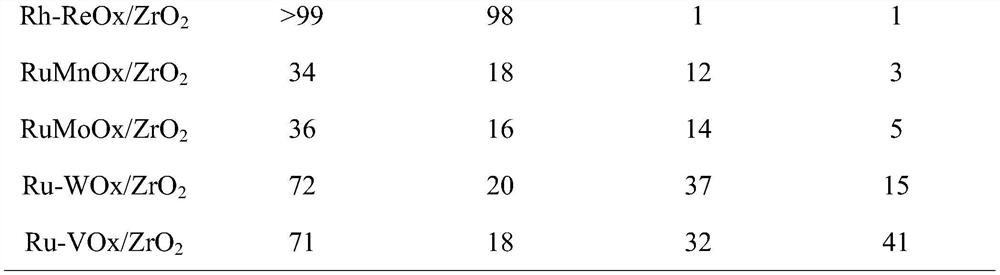
[0021] Table 1 Results of catalytic hydrogenolysis of 1,2,6-hexanetriol to 1,6-hexanediol on different active metal catalysts (160℃, 1MPa H 2 , The solvent is 2mL water, the amount of 1,2,6-hexanetriol is 5mmol, the loading amount of active component is 5wt%, the co-catalyst: active component=1, the amount of catalyst is 5wt%, the amount of phosphotungstic acid is 5wt %, the reaction time is 2h)
[0022]
[0023]
[0024] It can be seen from Table 1 that the best active component is Ru and the best cocatalyst is Re. Take Ru-ReOx / ZrO 2 When it is a catalyst, the substrate is completely converted, and the yield of 1,6-hexanediol is 97. Followed by Ru / ZrO 2 When it is a catalyst, the substrate is completely converted, and the yield of 1,6-hexanediol is 95%. Considering economic issues, the catalyst used in the following experiment is Ru / ZrO 2 .
Embodiment 3
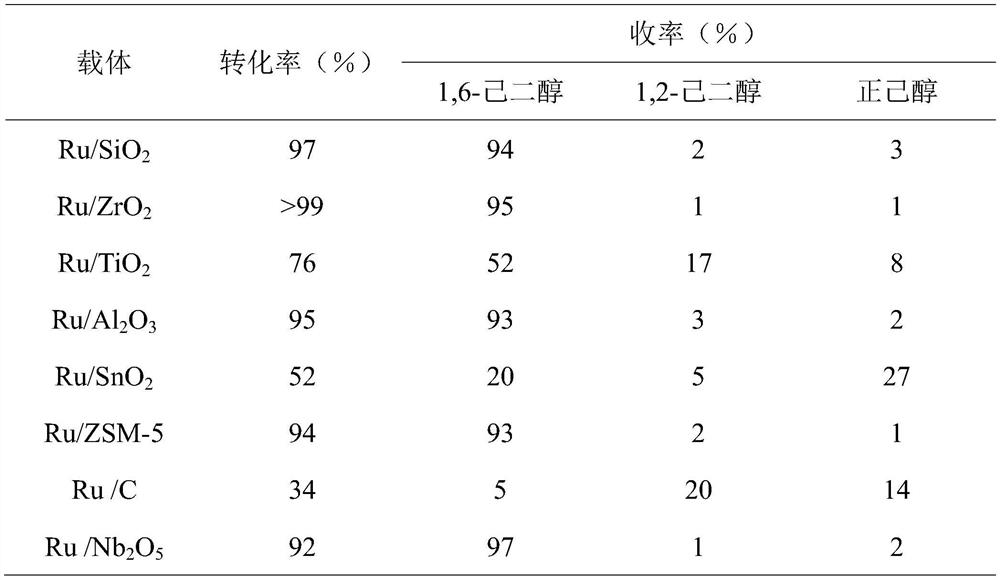
[0026] Table 2 Results of catalytic hydrogenolysis of 1,2,6-hexanetriol to 1,6-hexanediol on different catalyst supports (160℃, 1MPaH 2 , The solvent is 2mL water, the amount of 1,2,6-hexanetriol is 5mmol, the amount of Ru loading is 5wt%, the amount of catalyst is 5wt%, the amount of phosphotungstic acid is 5wt%, and the reaction time is 2h)
[0027]
[0028] It can be seen from Table 2 that the best carrier for the active component under the same reaction conditions is ZrO 2 At this time, 1,2,6-hexanetriol is completely converted, and the selective yield of 1,6-hexanediol is 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com