A method for preparing glycolic acid esters from carbohydrates
A carbohydrate and glycolic acid ester technology, applied in the preparation of organic compounds, chemical instruments and methods, and carboxylate preparation, etc., to achieve high-yield preparation, high selectivity, and convenient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
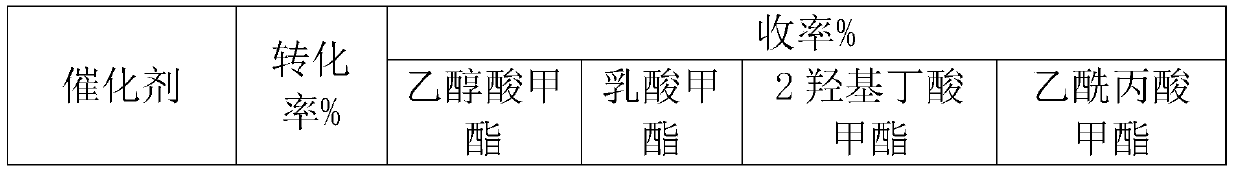
[0016] Cs 2.5 h 0.5 PW 12 o 40 Catalyst preparation
[0017] 2.5g CS 2 CO 3 and 18.12g of pretreated H 3 PW 12 o 40 6H20 formulated into 0.1mol L -1 and 0.08mol L -1 solution, 0.1mol.L -1 Cs 2 CO 3 Solution at 1mL·min -1 The rate is added dropwise to 0.08mol L at room temperature -1 h 3 PW 12 0 40 in solution. After the dropwise addition, continue to stir for 0.5h, then let it stand at room temperature for 20h. Then slowly evaporate the water at 323K to obtain a white solid, which is then dried at about 110°C and roasted at 300°C for 2 hours before use, with a specific surface area of 108m 2 g-1.
Embodiment 2
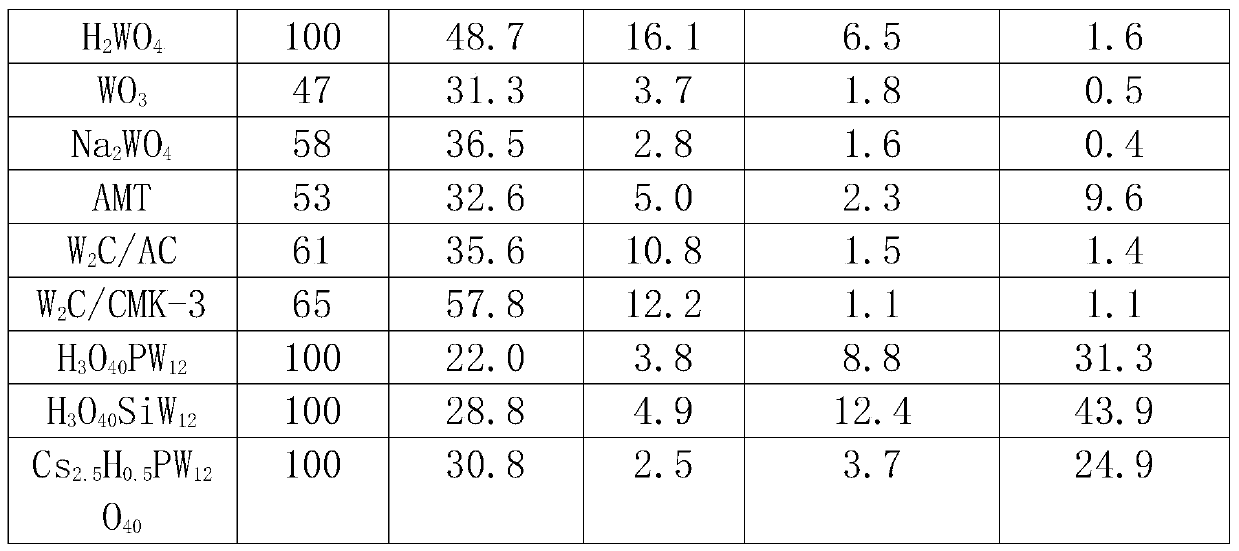
[0019] W 2 Preparation of C / AC: weigh 50g activated carbon (AC), 250mL 33wt% HNO 3 , placed in a 500mL three-neck flask, treated in a water bath at 80°C for 24h, washed until neutral, and dried at 120°C for 24h. Pour 1g of pretreated AC into an aqueous solution containing 0.588g of ammonium metatungstate, and after drying in an oven at 120°C, the catalyst precursor is subjected to temperature-programmed reduction in hydrogen. The specific reaction process is: from room temperature to 8.8°C The temperature was raised to 550 °C at a heating rate of 1 °C / min, and then to 900 °C at a heating rate of 1 °C / min and maintained for 1 h, with a hydrogen flow rate of 120 mL / min. The theoretical loading of W in the prepared catalyst is 30wt%.
Embodiment 3
[0021] W 2 Preparation of C / CMK-3: The preparation process was similar to Example 2, except that the AC carrier was replaced by CMK-3. The preparation process of CMK-3 is as follows: dissolve 1.25g sucrose in 5g deionized water, add 0.14g concentrated sulfuric acid, add 1g SBA-15 to the obtained mixed solution, soak at room temperature for 6h, and then dry at 100°C and 160°C for 6h respectively . The obtained beige powder was dipped again in a solution consisting of 0.8 g of sucrose, 0.09 g of concentrated sulfuric acid and 5 g of deionized water, and the drying step was repeated. Transfer to a tube furnace at a constant temperature of 900°C for 6 hours under a nitrogen atmosphere to completely carbonize the sucrose. Add 50ml of 4wt% hydrofluoric acid to the obtained black powder, stir at room temperature for 2h, filter / wash, repeat this process three times to completely remove silica, and finally dry overnight in an oven at 120°C to obtain ordered mesoporous carbon CMK -3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com