A kind of method for preparing ethylene glycol by catalytic conversion of carbohydrates
A carbohydrate and catalytic conversion technology, which is applied in the preparation of hydroxyl compounds, organic compounds, chemical instruments and methods, etc., can solve the problems of high technical difficulty, many by-products, low efficiency, etc., and achieve good cycle performance and corrosion resistance small, selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
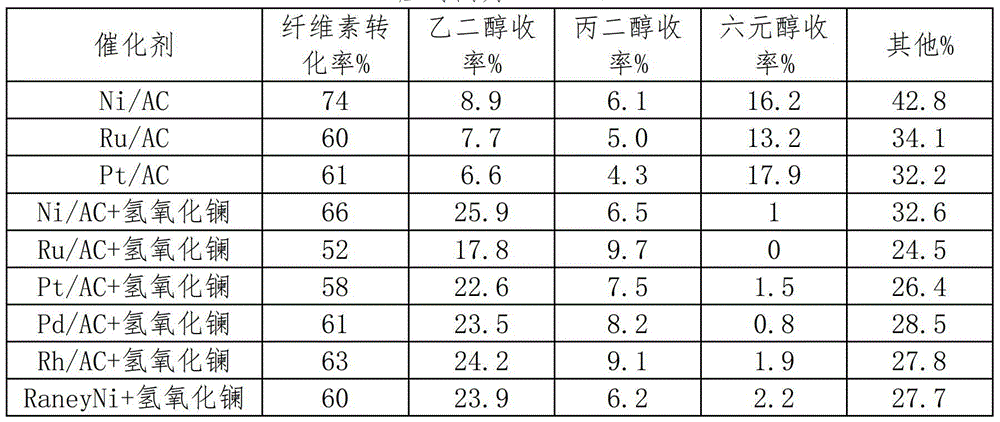
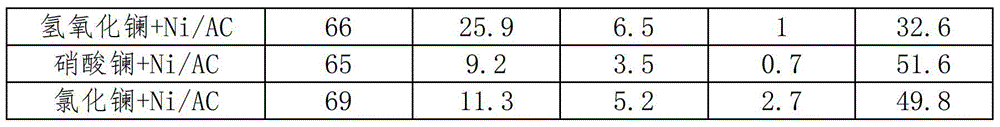
[0025] Preparation of lanthanum-based catalysts: non-supported catalysts such as lanthanum oxides, hydroxides, and salts are commercial drugs purchased directly, and the purity grades of the drugs are all analytically pure. In the supported catalyst, the active component is loaded on the carrier, and the carrier is a composite carrier of one or more of activated carbon, alumina, silicon oxide, silicon carbide, zirconia, zinc oxide, and titanium dioxide, and the metal salt of lanthanum (The anion can be one or more of nitrate, chloride or bromide) The aqueous solution is loaded onto the carrier by equal volume impregnation, dried overnight at 120°C, and then calcined at 700°C for 4h under N2 atmosphere.
Embodiment 2
[0027] Preparation of metal hydrogenation catalyst: use one or more of activated carbon, alumina, silicon oxide, silicon carbide, zirconia, zinc oxide, and titanium dioxide as a carrier, and chloroplatinic acid, palladium chloride, ruthenium chloride, and rhodium chloride The aqueous solutions of iridium chloride, nickel nitrate, iron nitrate and cobalt nitrate were respectively loaded on the carrier by equal volume impregnation method, and dried overnight at 120°C.
[0028] The above catalysts loaded with ruthenium, rhodium, palladium, iridium, platinum and other precious metals need to be reduced with hydrogen at 250°C for 2 hours and passivated for 4 hours under 1% O2 / N2 (V / V) atmosphere; , Cobalt and other non-noble metal catalysts need to be reduced with hydrogen at 450°C for 2 hours and passivated for 4 hours under 1% O2 / N2 (V / V) atmosphere before use.
Embodiment 3
[0030] Preparation of lanthanum oxide-supported nickel catalyst: Weigh 1.5g La2O3, 0.8g Ni(NO3)2·6H2O, dissolve nickel nitrate in 20ml water, then add La2O3 into the completely dissolved nickel nitrate solution, and place in a water bath at 25°C , and stirred for 12h until the solution evaporated completely. Dry in an oven at 120°C for 8h, calcinate at 500°C for 2h in N2 atmosphere, and then reduce for 5h at 500°C in H2 atmosphere.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com