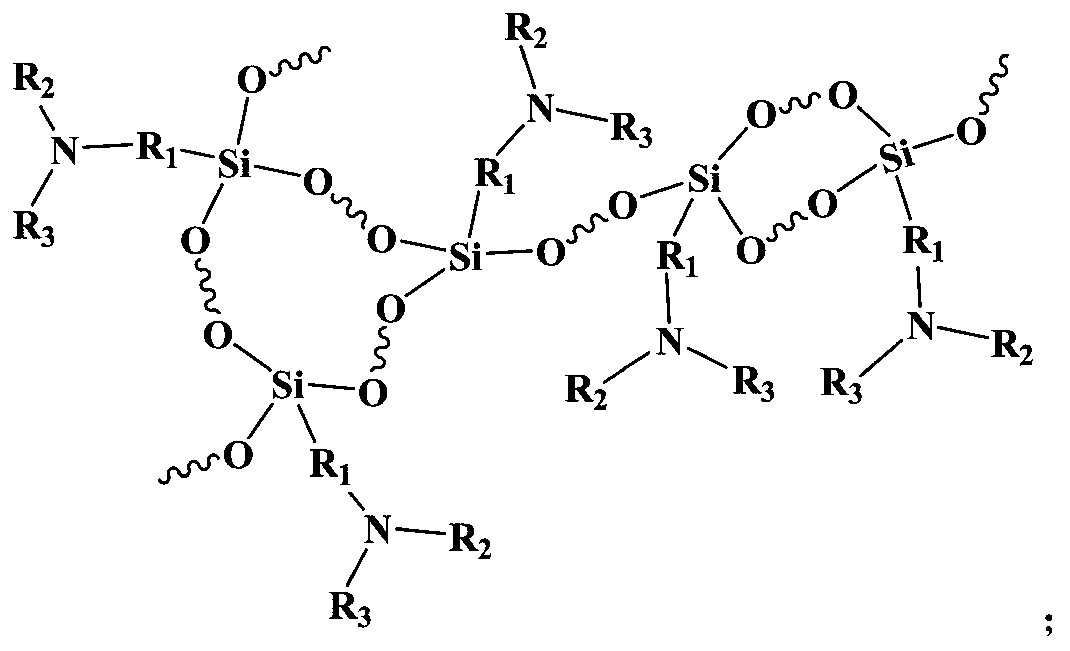
Crosslinked siloxane acrylate oligomer and photo-cured composition prepared from same
An acrylate and acrylate-based technology, applied in the field of light-curing polymer materials, can solve the problems of poor wiping resistance, poor anti-fouling ability, and poor inhibition of oxygen inhibition, and achieve fast curing speed and improved firmness. , Excellent inhibition of oxygen inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A crosslinked siloxane type acrylate oligomer, the preparation steps of which are as follows:
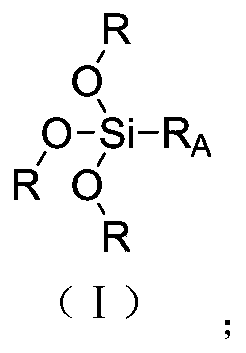
[0047] S1. Step: Preparation of the compound of formula (I), the compound of formula (I) in this embodiment is named as compound A 1 .
[0048] Add 18g of (3-piperazinepropyl)trimethoxysilane and 20g of trimethylolpropane triacrylate (TMPTA, technical grade purity) into 100mL of acetonitrile solution, under nitrogen protection, stir and react at 60°C for 12 h, The solvent was spin-dried to obtain off-white transparent liquid, and compound A was obtained 1 . Compound A 1 The structural formula is In ESI(+)-MS, (M+2) / Z=274, and in the infrared spectrum, at 3424cm -1 There is almost no infrared absorption at the (N-H bond stretching vibration absorption peak), which shows that the target compound A has been successfully synthesized. 1 .
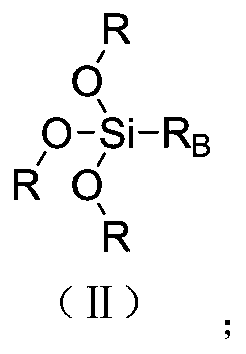
[0049] S2. Step: Compound A 1 Preparation of oligomer M by reaction and crosslinking with hydroxyl-terminated polysiloxane compound ...
no. 2 example
[0056] This embodiment is the second embodiment of the crosslinked siloxane type acrylate oligomer of the present invention, and its preparation steps are as follows:
[0057] S1. Step: Preparation of the compound of formula (I), the compound of formula (I) in this embodiment is named as compound A 2 .
[0058] Add 18g of N-(β-aminoethyl)-γ-aminopropyltrimethoxysilane and 53g of 1,6-hexanediol diacrylate (HDDA, technical grade purity) into 100mL acetonitrile solution, under nitrogen protection, After stirring and reacting at 50°C for 12h, the solvent was spin-dried to obtain an off-white transparent liquid, and compound A 2 . Compound A 2 The structural formula is In ESI(+)-MS, (M+1) / Z values are 451, 677, 903, and in the infrared spectrum, at 3427cm -1 There is almost no infrared absorption at the (N-H bond stretching vibration absorption peak), which shows that the target compound A has been successfully synthesized 2 .
[0059] S2. Step: Compound A 2 Preparation ...
no. 3 example
[0066] This embodiment is the third embodiment of the crosslinked siloxane type acrylate oligomer of the present invention, and its preparation steps are as follows:
[0067] S1. Step: Preparation of the compound of formula (I), the compound of formula (I) in this embodiment is named as compound A 3 .
[0068] Add 18g of N-n-butylaminopropyltrimethoxysilane and 13g of 1,2-ethylene glycol diacrylate (industrial grade purity) into 60mL of acetonitrile solution, under nitrogen protection, stir and react at 50°C for 12h, and then The solvent was spin-dried to obtain an off-white transparent liquid, and compound A was obtained 3 . Compound A 3 The structural formula is In ESI(+)-MS, the (M+1) / Z value is 306, and in the infrared spectrum, at 3430cm -1 There is almost no infrared absorption at (N-H bond stretching vibration absorption peak), which indicates that the target compound A was successfully synthesized 3 .
[0069] S2. Step: Compound A 3 Preparation of oligomer M b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com