Polyacyl urea elastomer and preparation method and application thereof
A polyurea and elastomer technology, applied in polyurea/polyurethane coatings, polyurea/polyurethane adhesives, adhesive types, etc., can solve the problem of isocyanate group water vapor sensitivity, free isocyanate monomer residual toxicity, etc. problem, achieve the effect of reducing energy use and shortening production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The first aspect of the present invention provides a kind of preparation method of polyurea elastomer, and this preparation method comprises: Polycarbodiimide, the polymer containing at least two acidic functional groups and optional chain extender, plasticizer , the catalyst and the solvent are uniformly mixed; then, curing is carried out to obtain the polyurea elastomer.
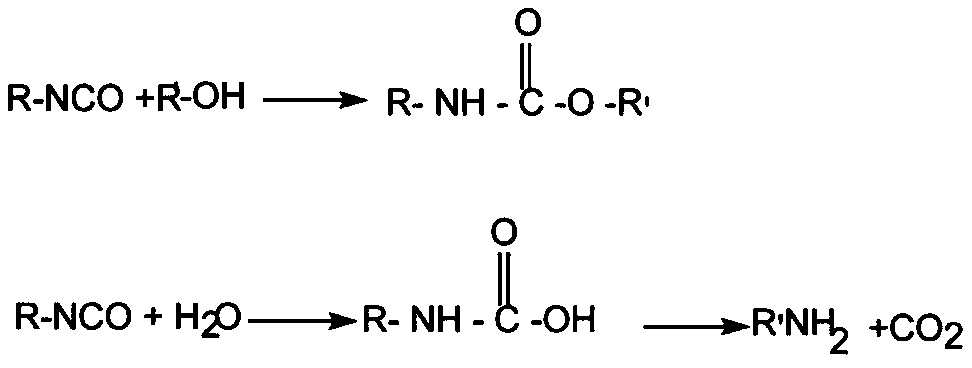
[0020] A polyacylurea is a polymer composed of acylurea linkages. It is obtained by reacting carbodiimide groups with carboxyl groups. The present invention uses isocyanate and its derivatives as the source of polycarbodiimide reactants, and in the presence of a catalyst, carbon dioxide is removed, NCO is converted into NCN, and then combined with polymers / chain extenders containing at least two acidic functional groups, etc. Cross-linking or chain extension. The generated acyl urea bonds are similar in structure to urethane bonds and amide bonds, have strong polarity, and are easy to form strong ...
Embodiment 1
[0051] Take 11.5 parts of polycarbodiimide in a three-necked bottle, add 5.3 parts of 150# solvent oil, and stir at room temperature for 2 hours; take 4 parts of dimer fatty acid (weight average molecular weight is 560), catalyst dibutyltin dilaurate 0.02 part , added to the polycarbodiimide solution under vigorous stirring, and continued to stir for 10 minutes. Pour it into the mold, and maintain it for 7 days under the standard environment of 23±2°C and humidity of 50±10%. Unmold and test.
[0052] Wherein, the polycarbodiimide is prepared by condensation reaction of toluene diisocyanate under the catalysis of organic phosphine, and is terminated with monocyanate; the weight average molecular weight of the polycarbodiimide is 1704.
Embodiment 2
[0054] Get 18.4 parts of polycarbodiimide in a three-necked bottle, add 5.3 parts of 150# solvent oil, 1 part of diisononyl phthalate (DINP), stir at normal temperature for 2 hours, add dimer fatty acid (weight average molecular weight 8 parts of 560), 1 part of trimerized fatty acid (weight average molecular weight is 900), 15 parts of No. 52 chlorinated paraffin, 6 parts of 150# solvent oil, and continued to stir for 10 minutes under strong stirring. Pour it into the mold, and maintain it for 7 days under the standard environment of 23±2°C and humidity of 50±10%. Unmold and test.
[0055] Wherein, the polycarbodiimide is prepared by the condensation reaction of diphenylmethane diisocyanate under the catalysis of an organic phosphine, and is terminated with a monocyanate; the weight average molecular weight of the polycarbodiimide is 1360.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com