Fusion protein, and pharmaceutical composition and use thereof
A technology of fusion protein and composition, applied in the field of fusion protein, can solve problems such as difficult to use and short half-life, and achieve the effect of promoting cartilage repair, long half-life, and promoting new bone formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of Example 1 AC-100 Peptide
[0040] AC-100 peptide (SEQ ID NO: 1) was entrusted to Shanghai Aobo Biotechnology Co., Ltd. to synthesize by conventional solid-phase technology, and the purity of the synthesized peptide was >98%.
Embodiment 2
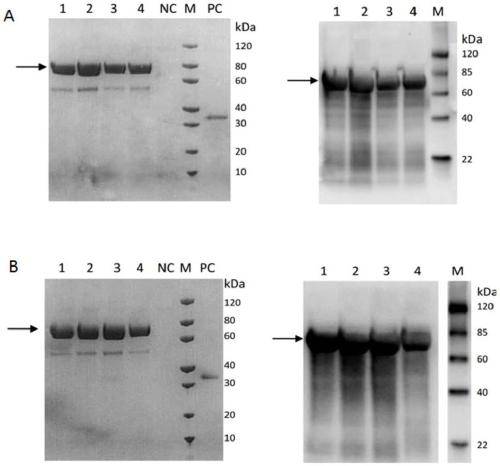
[0041] Construction and expression of embodiment 2 recombinant fusion protein strain
[0042] The construction of recombinant expression strains of fusion proteins HSA021-1 and HSA021-2 was entrusted to Nanjing GenScript Biotechnology Co., Ltd. The main steps are: synthesizing the target gene (SEQ ID NO: 2, corresponding to the nucleotide sequence encoding HSA021-1, which contains the sequence encoding the signal peptide; SEQ ID NO: 4, corresponding to the nucleotide sequence encoding HSA021-2 sequence, which contains the sequence encoding the signal peptide), was connected to the expression yeast expression vector pPICZaA through the same digestion by XhoI / NotI double digestion, and 10 μg of the expression vector after the connection was used to transfect Pichia pastoris (Pichia pastoris) X-33 , PCR identified positive clones. Selected positive clones were cultured in BMGY medium, OD 600 When it reached 3.0, the cells were harvested, resuspended in BMGY medium, and induced ...
Embodiment 3
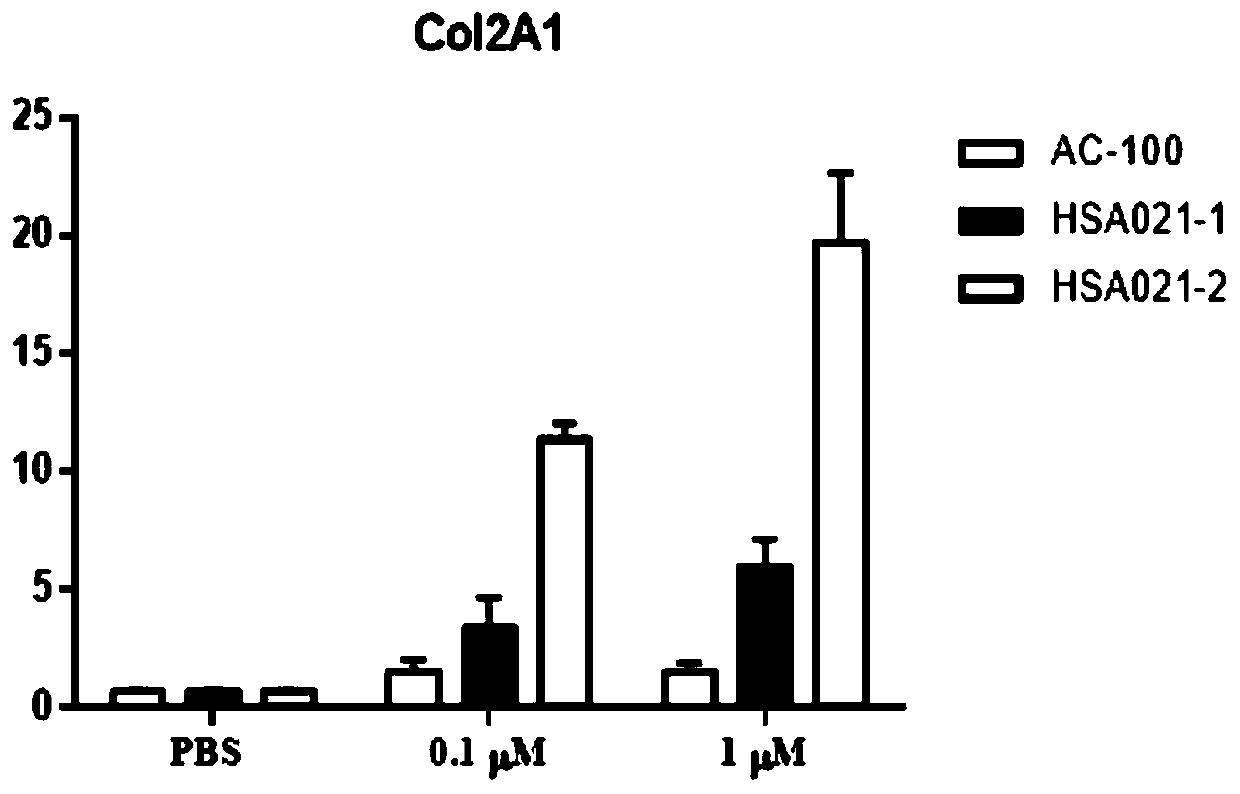
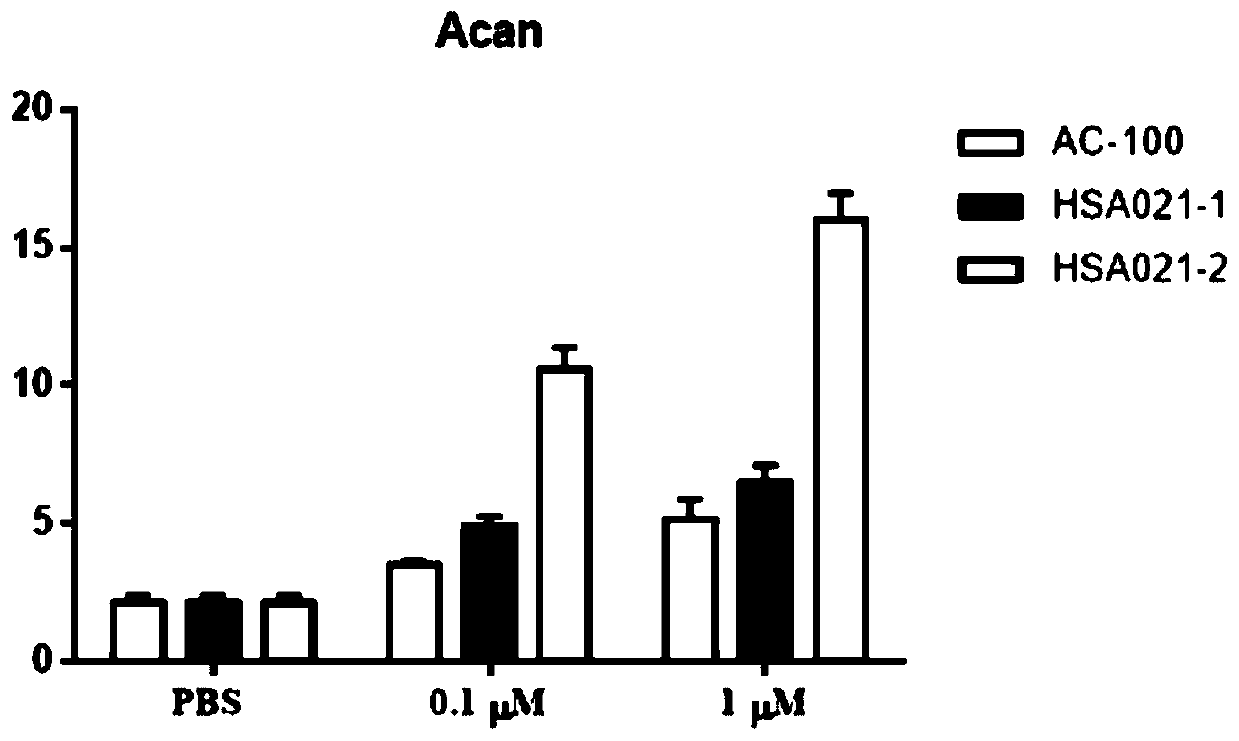
[0044] Example 3 Effects on Bone Marrow Mesenchymal Stem Cells (BMSCs)
[0045] Three 8-week-old SD rats were killed by dislocation. After being sterilized with 70% ethanol, the abdominal cavity was cut open and the femur was separated. Use a syringe to draw low-sugar DMEM culture solution (containing 1 mL / L heparin) to flush the bone marrow cavity repeatedly to flush out the bone marrow.
[0046] The bone marrow fluid was made into a single-cell suspension, and DMEM-F12 complete medium containing 10% FBS (fetal bovine serum, fetal bovine serum) was added, and 10 7 The density of each / mL was inoculated in a culture flask, placed at 37°C, 5% CO 2 Cultivate in an incubator, change the culture medium after 48 hours, and then change the culture medium every 3 days to remove non-adherent hematopoietic cells. When the cells reach 80% to 90%, subculture, add the complete culture medium of mesenchymal stem cells to resuspend the cells, and store at 37°C, 5% CO 2 cultured in an incu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com