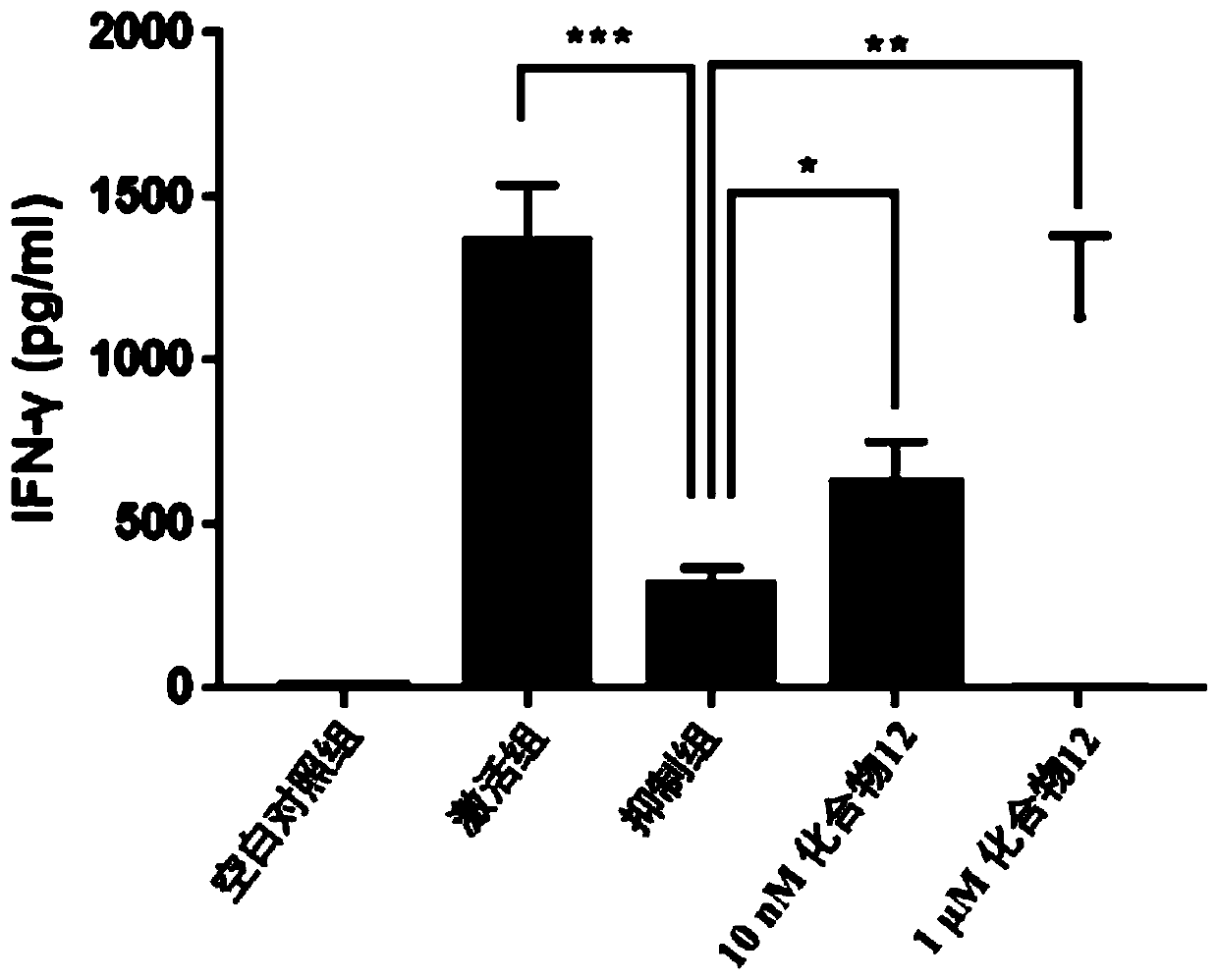
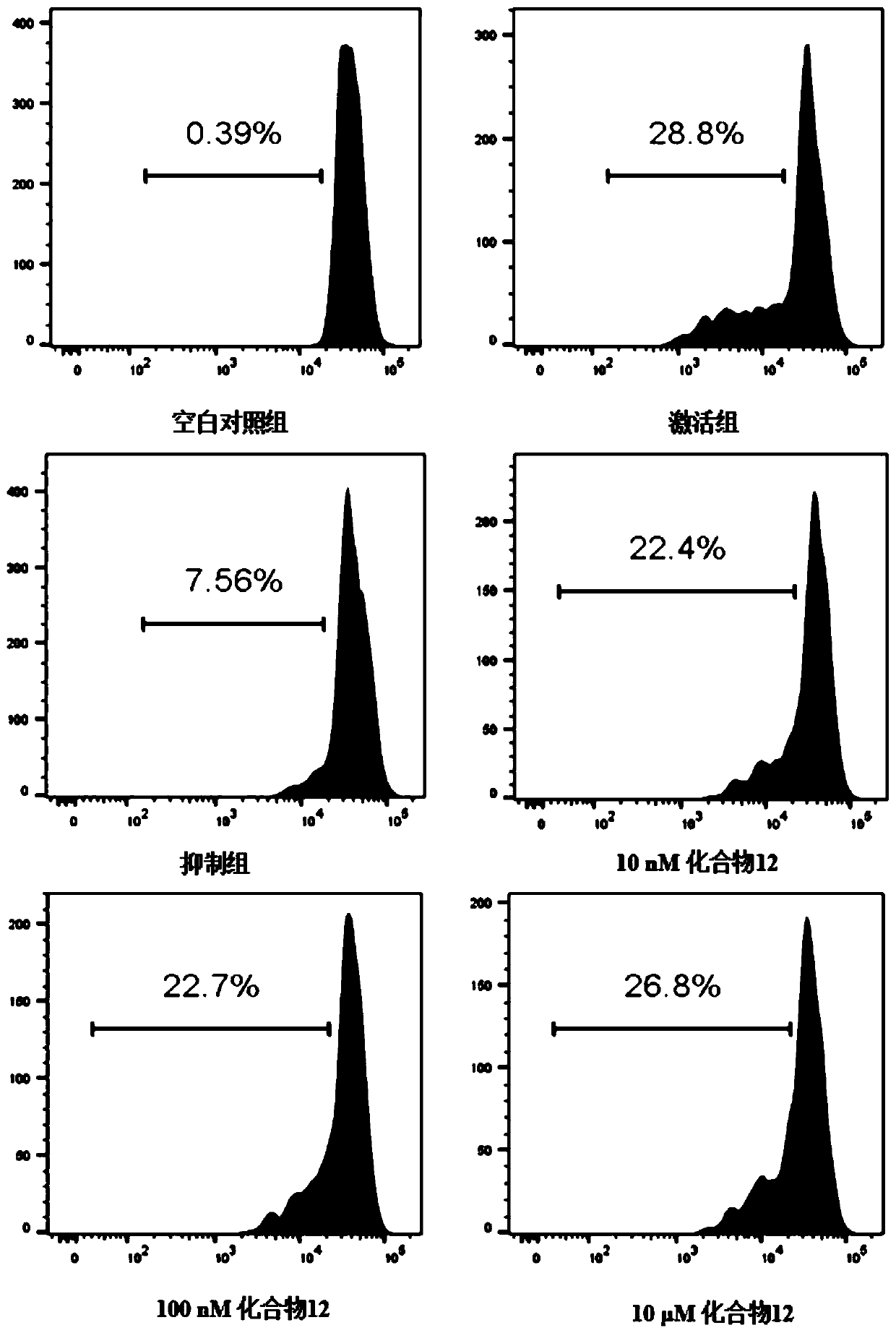
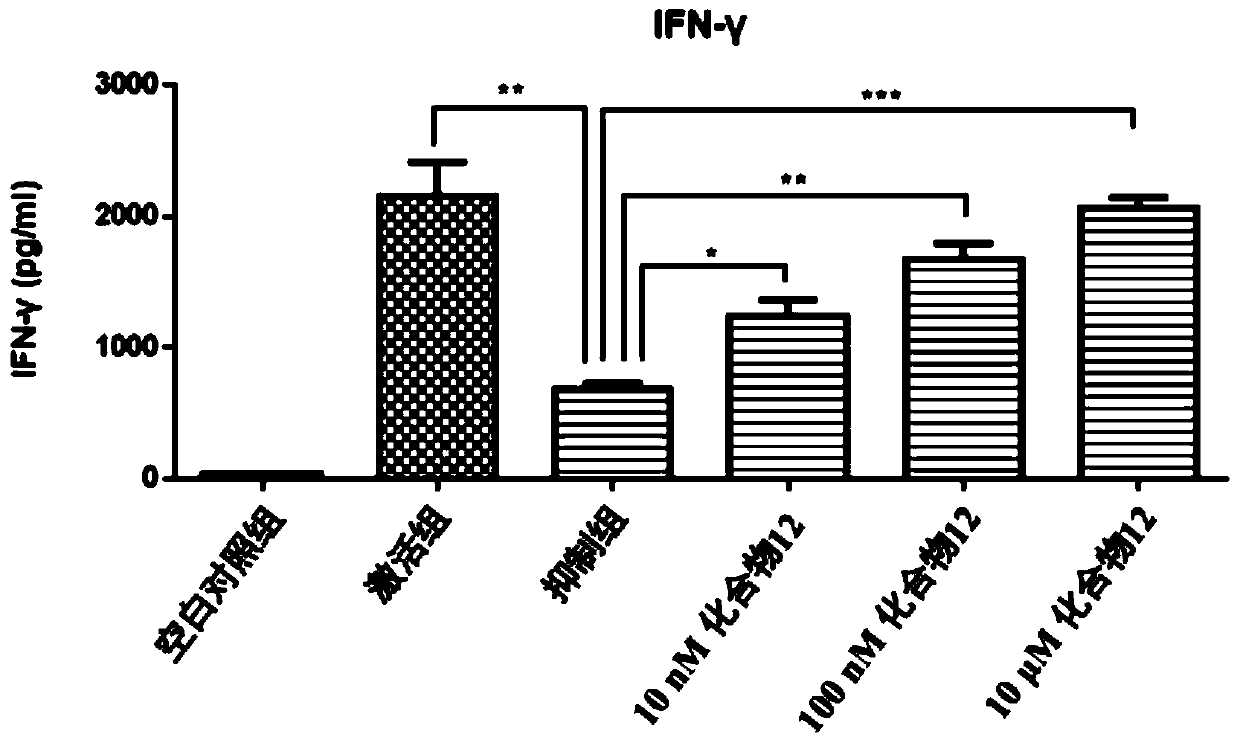
Benzoxadiazole compound and preparation method and medical use thereof
A technology of benzoxadiazoles and compounds, applied in the field of benzoxadiazoles, pharmaceutical compositions for tumor immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] N-((5-((3-cyanobenzyl)oxy)-7-((2-methyl-[1,1'-biphenyl]-3-yl)methoxy)benzo[c ][1,2,5]oxadiazol-4-yl)methyl)-D-serine (compound 1)
[0110]
[0111] synthetic route:
[0112]
[0113] Synthesis of Compound I-2
[0114] Dissolve compound M-2 (1.8g) in anhydrous acetone (30mL), add anhydrous sodium bicarbonate (1.3g) and tetrabutylammonium iodide (TBAI, 300mg), and slowly add I-1 (2g ), protected by argon, and heated in an oil bath at 70°C. After reacting for about 6 hours, the heating was stopped, the solvent was evaporated under reduced pressure, diluted with ethyl acetate (25 mL), washed with water (10 mL x3) and saturated brine (10 mL x2), and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the residue was purified by column chromatography (eluent: petroleum ether / ethyl acetate=3:1) to obtain compound I-2 (2.14 g) as a light yellow solid.
[0115] Synthesis of Compound I-3
[0116] Compound I-2 (2.33 g) was dissolv...
Embodiment 2
[0128] N-(2-(((5-((3-cyanobenzyl)oxy)-7-((2-methyl-[1,1'-biphenyl]-3-yl)methoxy) Benzo[c][1,2,5]oxadiazol-4-yl)methyl)amino)ethyl)acetamide (compound 2)
[0129]
[0130] Referring to the method of Example 1, the D-serine ethyl ester hydrochloride in Example 1 was replaced by N-acetylethylenediamine to obtain compound 2: 1 H NMR (300MHz, CDCl 3 )δ7.73(s,1H),7.71–7.60(m,2H),7.54(t,J=7.7Hz,1H),7.40(m,J=13.3,6.7Hz,4H),7.29(m,4H ),6.58(s,1H),6.28(s,1H),5.38(s,2H),5.21(s,2H),4.09(s,2H),3.34(dd,J=10.8,5.3Hz,2H) ,2.74(t,J=5.6Hz,2H),2.28(s,3H),1.95(s,3H).HRMS(ESI):m / z 562.24496[M+H] + .
Embodiment 3
[0132] N-((5-((3-cyanobenzyl)oxy)-7-((2-methyl-[1,1'-biphenyl]-3-yl)methoxy)benzo[c ][1,2,5]oxadiazol-4-yl)methyl)glycine (Compound 3)
[0133]
[0134] Referring to the method of Example 1, the D-serine ethyl ester hydrochloride in Example 1 was replaced with glycine methyl ester hydrochloride to obtain compound 3: 1 H NMR (300MHz, CD 3 OD)δ7.95–7.83(m,2H),7.75–7.54(m,3H),7.50–7.21(m,7H),6.96(s,1H),5.44(s,2H),5.40(s,2H ),4.05(s,2H),3.18(s,2H),2.27(s,3H).MS(ESI):m / z 533.3[M-H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com