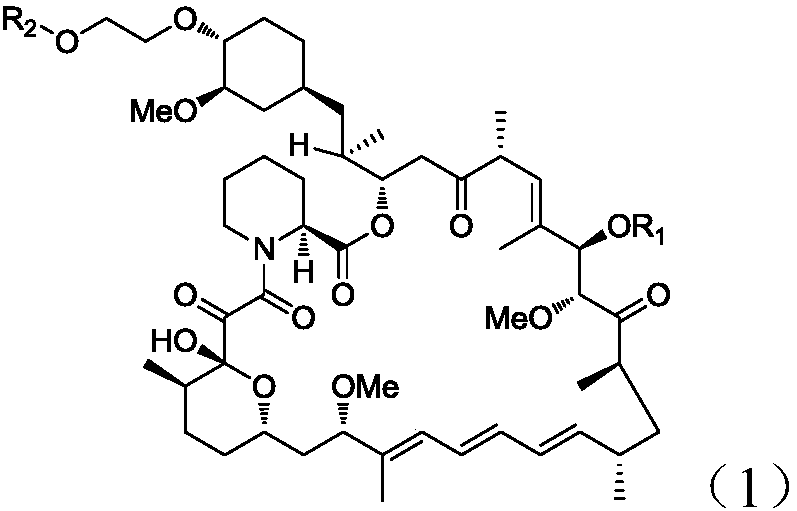
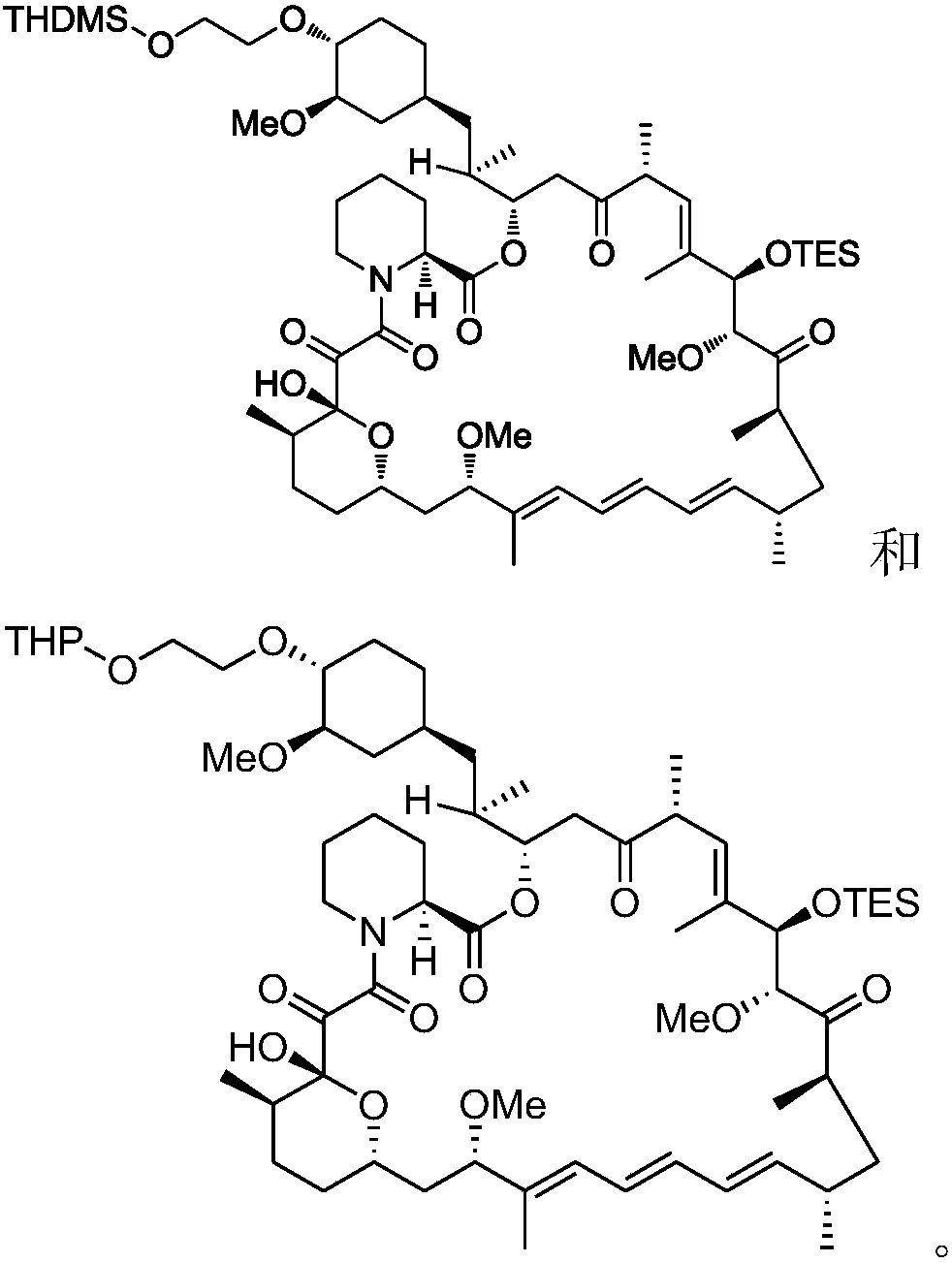
Everolimus intermediate, and preparation method and application thereof
A technology for everolimus and intermediates, applied in the field of everolimus intermediates, can solve problems such as difficult separation, many by-products, and many impurities, and achieve the effects of reducing side reactions, simple preparation methods, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] (1), the synthesis of 28-O-TMS rapamycin (intermediate B-1)
[0064]
[0065] Dissolve rapamycin (5.00g) and imidazole (1.86g) in ethyl acetate (100mL), under cooling in an ice-water bath, add trimethylchlorosilane (2.97g) dropwise, and continue cooling in an ice-water bath after the addition is complete The reaction was stirred for 30 minutes, and TLC showed that the starting material disappeared completely.
[0066] Add 0.5M hydrochloric acid (8mL) under ice-water bath and continue to cool, and stir the reaction. TLC shows that the original small polar product completely disappears, and the polarity of the main product is between rapamycin and rapamycin. The reaction was stopped, the reaction liquid was separated, the organic phase was washed with saturated sodium bicarbonate and saturated brine successively, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 28-O-TMS rapamycin (intermediate B-1, 5.89 g), as a white solid. ESI-...
Embodiment 2
[0074] In this embodiment, the reaction process is as follows:
[0075]
[0076] (1), the synthesis of 28-O-TES rapamycin (intermediate B-2)
[0077]
[0078] Dissolve rapamycin (5.00g), triethylamine (2.77g) and 4-dimethylaminopyridine (20mg) in dichloromethane (100mL), and add triethylchlorosilane (4.12 g), after the dropwise addition was completed, reacted for 1 hour under cooling in an ice-water bath, then gradually warmed up to room temperature, and reacted overnight. TLC showed that the raw materials disappeared completely. The reaction solution was filtered, and the filtrate was washed successively with 0.5M dilute hydrochloric acid, saturated sodium carbonate and saturated sodium chloride, and the organic phase was concentrated under reduced pressure.
[0079] The obtained residue was dissolved in acetone (100ml), and 0.25M dilute sulfuric acid (15mL) was added under cooling in an ice-water bath, and the reaction was stirred overnight at 0-10°C. TLC showed that ...
Embodiment 3
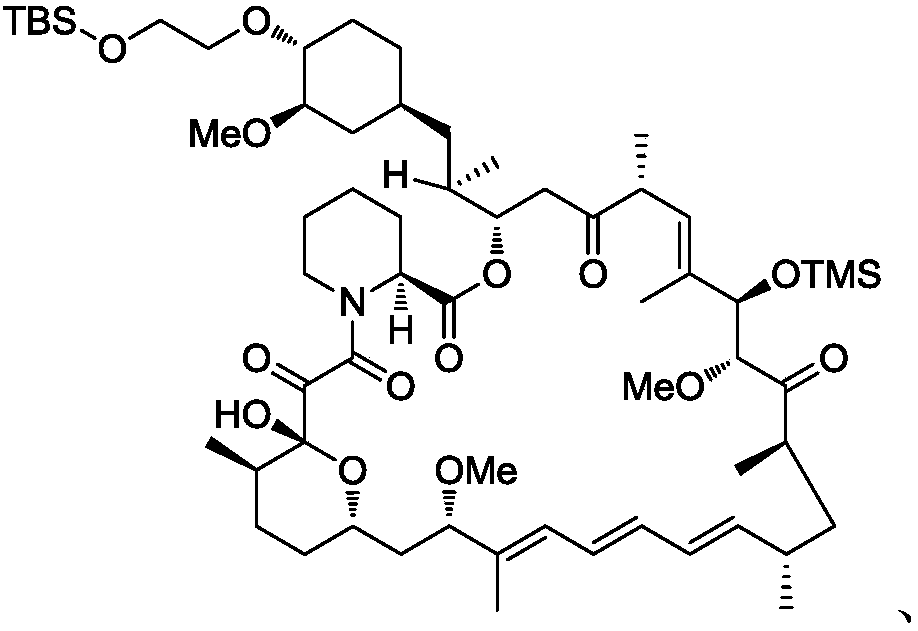
[0087] Synthesis of 28-O-TMS-42-O-TBS Everolimus (Intermediate C-1)
[0088]
[0089]Dissolve 28-O-TMS rapamycin (3.04g), N,N-diisopropylethylamine (3.00g) in toluene (20ml) and ethylene glycol dimethyl ether (4ml), add trifluoro 2-(tert-butyldimethylsilyloxy)ethyl methanesulfonate (4.45g) was heated at 60°C for 4 hours. The reaction solution was cooled and concentrated under reduced pressure, and the residue was subjected to column chromatography to obtain 28-O-TMS-42-O-TBS everolimus (compound 1, 2.42 g, yield 72.7%) as a white solid. 1H NMR (400MHz, CDCl 3 )δ: 0.00(m,15H),0.65-0.98(m,15H),1.05(m,9H),1.13-2.08(m,28H),2.2.-2.40(m,2H),2.75-2.90(m ,2H),3.01(1H),3.13(m,3H),3.25(m,3H),3.26-3.43(m,4H),3.50-3.89(m,8H),4.05-4.44(m,1H), 5.00-5.66 (m, 4H), 5.85-6.45 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com