A kind of cyclic polypeptide for resisting Candida albicans and its preparation method
A cyclic polypeptide and cyclic technology, applied in the field of antibacterial agents, can solve the problems of life and health hazards of patients, and achieve the effects of improving polypeptide stability, good antibacterial effect and low MIC value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The method of the present invention adopts the conventional solid-phase Fmoc method, that is, the monomer amino acid protected by Fmoc on the solid-phase resin is deprotected to expose the amino group, and forms a peptide bond with the carboxyl group of the amino acid in the solution through a condensation reaction, thereby linking the amino acid to the On the resin, the peptide chain is extended from the C-terminus to the N-terminus.
[0025] 1. Basic materials:
[0026] (1) Resin and linking molecule: The resin selected by the solid-phase Fmoc method is Rink Amide- resin. This kind of resin has very good swelling property, which can make the condensation reaction between the peptide chains better, and there is enough network space to meet the growing peptide chains. HBTU and HOBt are used as linking molecules to immobilize the polypeptide molecules on the resin.
[0027] (2) Monomer: The monomer used in the synthesis is a chemically modified α-amino acid.
[0028...
Embodiment 2
[0044] In the fourth step, repeat the second and third steps, add monomer amino acids repeatedly, according to LE 46 RRCR D PPFRCWE 46 The sequence of RRRRRRR sequences is synthesized from right to left until the synthesis is complete.
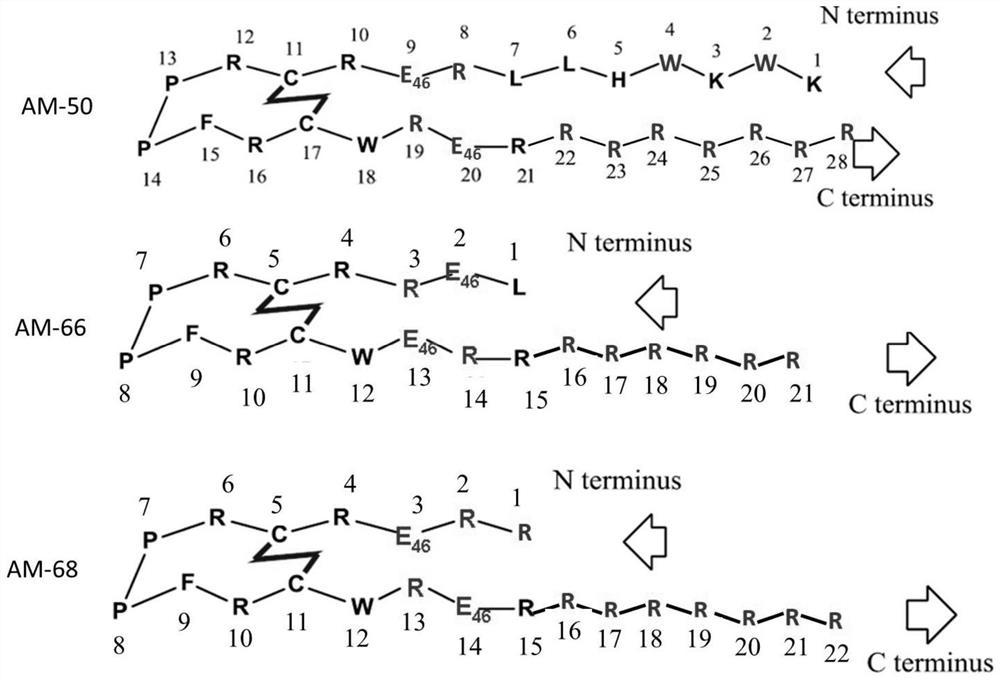
[0045] Other steps are the same as in Example 1, and the polypeptide sequence AM-66 ( figure 1 ).
[0046] The MIC value of the synthesized cyclic polypeptide is 4.46.
Embodiment 3
[0048] In the fourth step, repeat the second and third steps, add monomer amino acids repeatedly, according to RRE 46 RCR D PPFRCWRE 46 The sequence of RRRRRRR sequences is synthesized from right to left until the synthesis is complete.
[0049] Other steps are the same as in Example 1, and the polypeptide sequence AM-68 ( figure 1 ).
[0050] The MIC value of the synthesized cyclic polypeptide is 4.21.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com