A composition containing flupirtine or a pharmaceutically acceptable salt thereof and a preparation method thereof
The technology of a composition and flupirtine is applied in the field of compositions containing flupirtine or its pharmaceutically acceptable salts and its preparation, which can solve the problems of poor fluidity of raw materials, and avoid uneven coating or particle surface adhesion, Sustained-release preparations are safe and the effect of simplifying the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
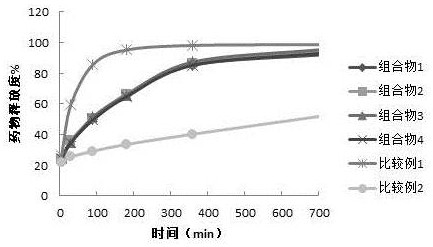
Embodiment 1
[0028] Example 1 Preparation of a composition containing flupirtine or a pharmaceutically acceptable salt thereof
[0029] Composition 1
[0030] 1. Preparation of sustained-release part: sieve flupirtine maleate, Eudragit RL100, pregelatinized starch, yellow iron oxide and talcum powder in Table 1, and mix the above-mentioned components in the prescription amount in Table 1 Uniform, use 5% hydroxypropyl cellulose aqueous solution as binder to prepare soft material, granulate with 30 mesh sieve, dry the granules prepared by wet method at 60°C until the moisture content is lower than 3.0%;
[0031]2. Mix the flupirtine maleate of the prescription amount in Table 2 and the flupirtine maleate sustained-release granules prepared above, and then add the croscarmellose sodium and stearin of the prescription amount in Table 2 Magnesium acid and micropowder silica gel are mixed evenly;
[0032] 3. Determining the content of the blended granule intermediate, submitting the pressure, ...
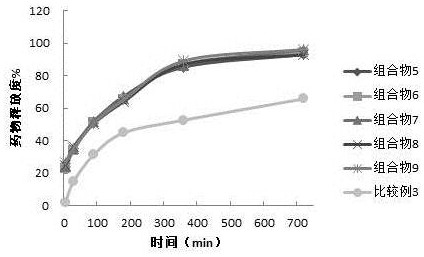
Embodiment 2
[0056] The selection test of embodiment 2 disintegrating agent
[0057] Sustained-release granules composition:
[0058]
[0059] Preparation method of sustained-release granules: sieve flupirtine maleate, methyl cellulose and yellow iron oxide, mix the above-mentioned ingredients evenly, use Eudragit NE30D as a binder to prepare a soft material, 30 Granulate with a mesh sieve, and dry the granules prepared by the wet method at 60°C until the moisture content is less than 3.0%.
[0060] Composition 5
[0061] Mix the flupirtine maleate and flupirtine maleate sustained-release granules in the prescription amount in Table 5 evenly, then add the CMS-Na, pregelatinized starch, magnesium stearate and micropowder silica gel in the prescription amount in Table 5 Mix evenly; measure the content of the blended granule intermediate, submit the pressure, compress the tablet, and obtain the sample.
[0062] Composition 6
[0063] The difference from composition 5 is that each compo...
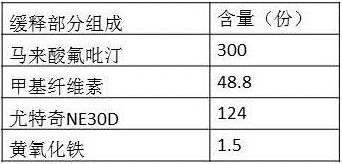
Embodiment 3
[0081] This embodiment examines the influence of magnesium stearate and micropowder silica gel on the pharmaceutical composition described in this application, and the formulation and preparation of sustained-release granules are as follows:
[0082]
[0083] Sustained-release granule preparation method: Sustained-release granule preparation method: sieve flupirtine maleate, methylcellulose and yellow iron oxide, mix each component of the above prescription amount evenly, use Eudragit NE30D as a binder Prepare soft materials with 30-mesh sieve, and dry the wet-processed granules at 60°C until the water content is lower than 3.0%.
[0084] The specific implementation is as shown in Table 8:
[0085] Composition 10
[0086] Mix the flupirtine maleate and the flupirtine maleate sustained-release granules of the prescription amount in Table 8 evenly, then add the croscarmellose sodium, pregelatinized starch, and stearin of the prescription amount in Table 8 Magnesium acid and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com