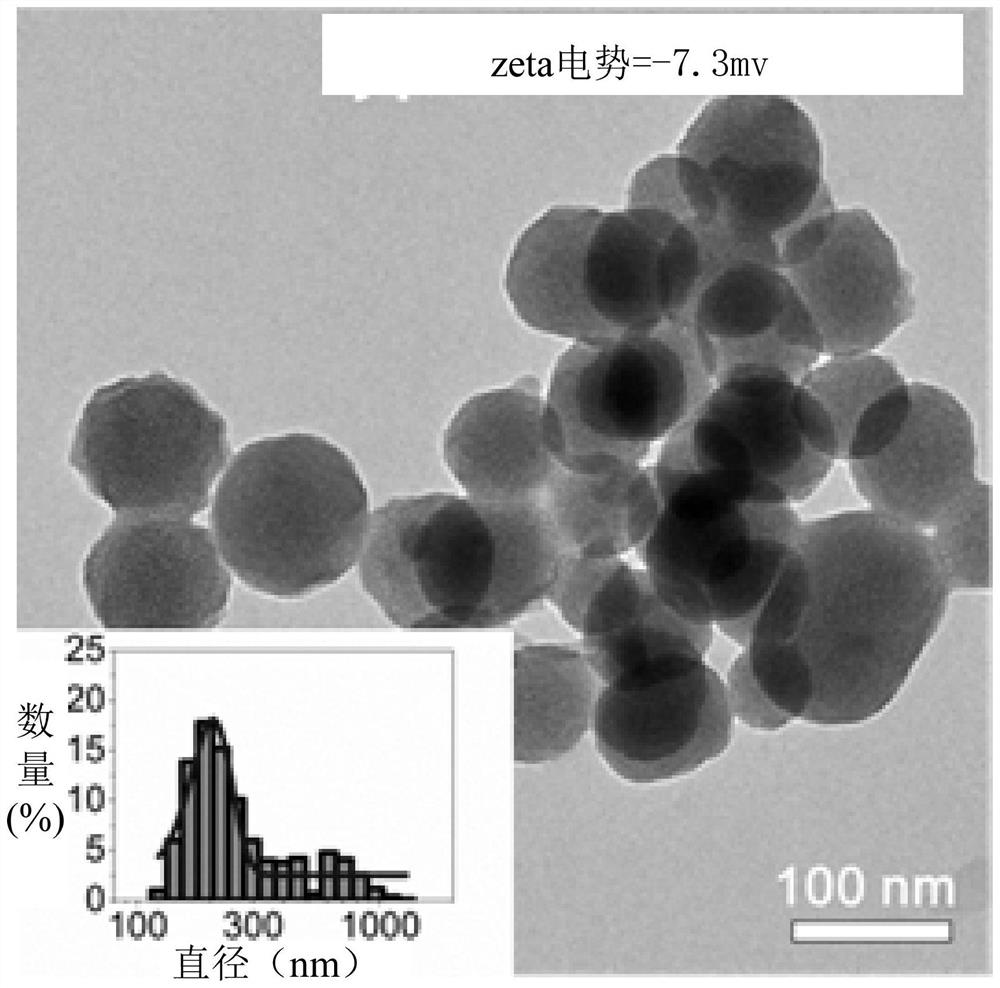
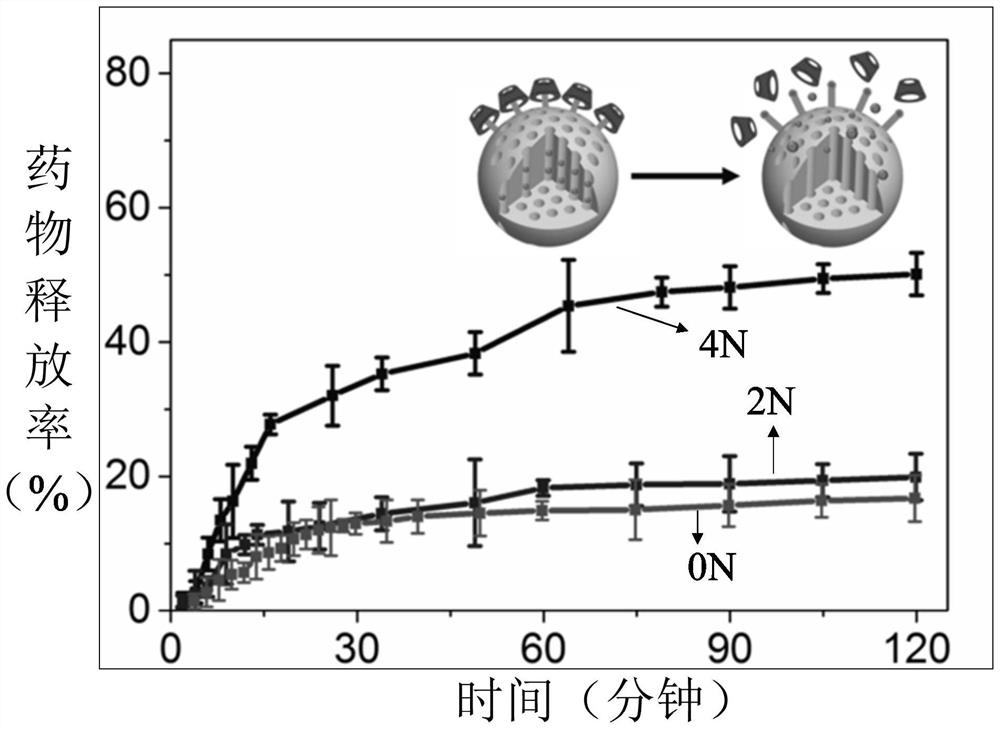
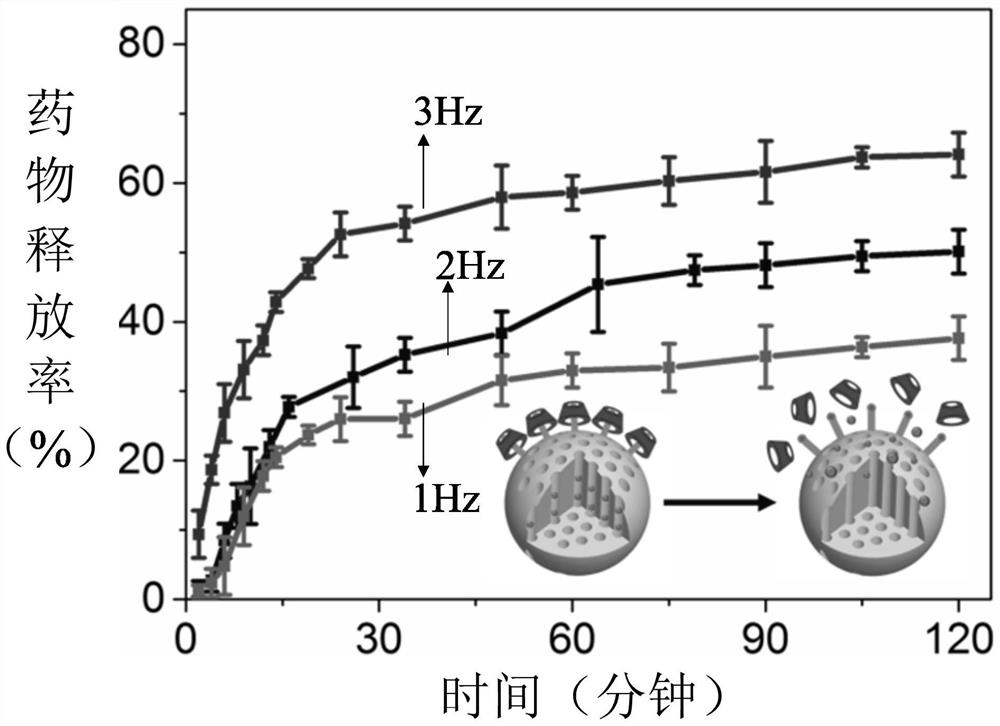
Sustained-release drug, its preparation method and application
A slow-release drug and drug technology, applied in the field of biomedicine, can solve problems such as inapplicable bones and joints
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The embodiment of the present invention also provides a preparation method of the sustained-release drug, comprising:
[0051] S10, providing the mesoporous silicon spheres;
[0052] S20, binding the guest molecule on the outer surface of the mesoporous silica sphere;
[0053] S30, loading the drug in the mesopore; and
[0054] S40, combining the host molecule with the guest molecule through host-guest interaction and at least partially blocking the opening of the mesopore.
[0055] In step S10, the mesoporous silicon spheres can be prepared by a template extraction method. In one embodiment, before step S20, it includes: modifying the mesoporous silicon spheres with amino groups. The amino modification step can be performed during the preparation of the mesoporous silicon spheres.
[0056] In one embodiment, the preparation of the mesoporous silicon spheres may include:
[0057] S12, mixing the silicon source, the templating agent and the morphology control cataly...
Embodiment 1
[0090] (1) Add 1 g of cetyltrimethylammonium bromide (CTAB) and 3.5 mL of sodium hydroxide solution (2 mol / L) into 480 mL of deionized water to obtain a mixed solution, and stir at 80°C for 30 min. 5 mL tetraethyl orthosilicate (TEOS) was added dropwise to the mixture, and stirring was continued at 80° C. for 2 h. Centrifuge, wash and dry in vacuo to obtain silica spheres.
[0091] (2) Disperse 1 g of the product of step (1) in 100 mL of anhydrous toluene, add 5 mL of 3-aminopropyltriethoxysilane (APTES), and reflux at 110° C. for 24 h. Centrifuge, wash and vacuum dry to obtain amino-modified silica spheres.
[0092] (3) Disperse 1 g of the product of step (2) in 100 mL of methanol, add 1 mL of concentrated hydrochloric acid, reflux at 60 ° C for 24 h, centrifuge, wash and dry in vacuum to obtain amino-modified mesoporous silica spheres (MSN-NH 2 ).
[0093] (4) Add 1.2 mL of thionyl chloride dropwise to 0.9 g of betaine hydrochloride, react at room temperature until no gas...
Embodiment 2
[0100] (1)-(3) Same as in the example, prepare amino-modified mesoporous silicon spheres.
[0101] (4) Dissolve 27g of cyclodextrin in 150mL of deionized water, add 9mL of NaOH solution (5.8mol / L) dropwise to the cyclodextrin solution, stir at room temperature for 30min, and the solution turns light green. Add 13 mL of acetonitrile solution of p-toluenesulfonyl chloride (1.58 mol / L) dropwise to the light green solution, and stir at room temperature for 3 h. Remove the precipitate by suction filtration, add hydrochloric acid (2mol / L) dropwise to the supernatant until the pH is 7.5, and place in the refrigerator overnight. The precipitate was collected by suction filtration and recrystallized three times with deionized water to obtain OTs group-modified cyclodextrin (OTs-CD).
[0102] (5) 100mg MSN-NH 2 Disperse into 20mL N,N-dimethylformamide (DMF), add 50mg OTs-CD and 2mL ethylenediamine (acid-binding agent), and stir at 110°C for 24h. Centrifuge, wash and dry in vacuum to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com