Bone-marrow-derived osteoclast culturing method
A technology derived from osteoclasts and bone marrow, applied in the direction of bone/connective tissue cells, animal cells, vertebrate cells, etc., can solve the problems of low purity of mononuclear cells of osteoclasts, many induction factors, and cumbersome steps. Achieve the effect of saving energy, simplifying the experimental process, and streamlining the experimental steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1. Configuration of complete medium
[0054] It is made by adding 15% fetal bovine serum FBS and 1% separating liquid double antibody solution to MEM Alpha basic medium.
[0055] 2. Isolation and culture of mouse bone marrow mononuclear cells
[0056] The 5-week-old ICR mice were killed by dislocation, soaked in 75% alcohol for 5 minutes, and the femur and tibia of the mouse were taken under aseptic conditions, and the bone marrow in the inner cavity was washed out by drawing 6ml of homogenate flushing solution with a 1mL syringe. Until the bone marrow cavity turns white. Filter the resulting cell suspension through a 100 μm cell mesh into a 10 mL centrifuge tube. Centrifuge at 450g for 10min. Discard the supernatant and add 0.6ml sample diluent to resuspend the cells. Take a 5mL glass blood collection tube, carefully add 4mL of separation solution 1, 1.33mL of separation solution 2 and cell suspension in sequence to form a gradient interface, and the liquid layer m...
Embodiment 2
[0060] 1. Configuration of complete medium
[0061] It is made by adding 10% fetal bovine serum FBS and 1% separating liquid double antibody solution to MEM Alpha basic medium.
[0062] 2. Isolation and culture of mouse bone marrow mononuclear cells
[0063] Take the femur and tibia of two 5-week-old ICR mice, and use a 1 mL sterile syringe to draw an appropriate amount of serum-free α-MEM culture solution to wash out the bone marrow in the inner cavity until the bone marrow cavity turns white. Pipette repeatedly to form a cell suspension, filter through a 100μm cell mesh into a 10mL centrifuge tube. Centrifuge at 1200r / min for 5min. Discard the supernatant, add 5mL red blood cell lysate, mix gently by pipetting, lyse on ice for 5min, and centrifuge at 1000r / min for 5min. Discard the supernatant, add 5mL 10% FBS, mix the cells, and centrifuge at 1000r / min for 5min. After discarding the supernatant, add an appropriate amount of 10% FBS to resuspend the cells, pipette evenly...
Embodiment 3
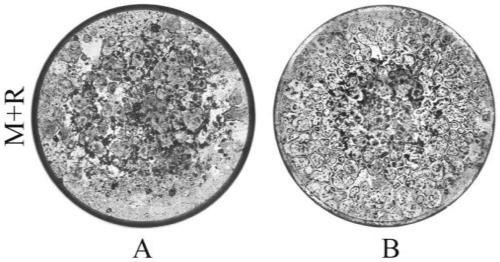
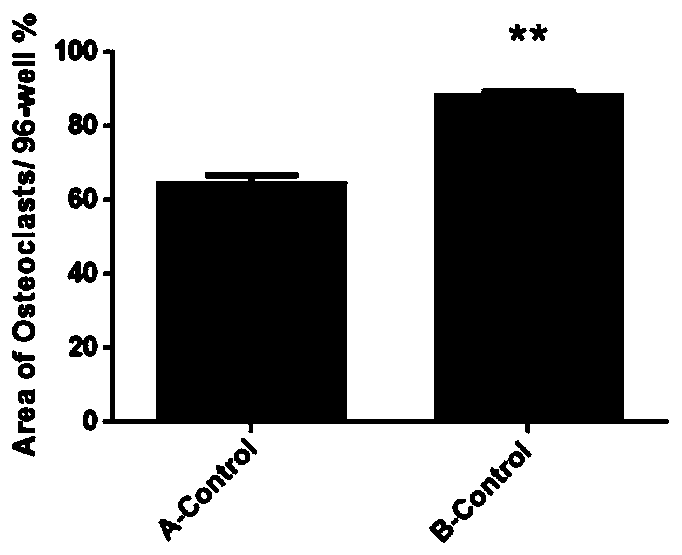
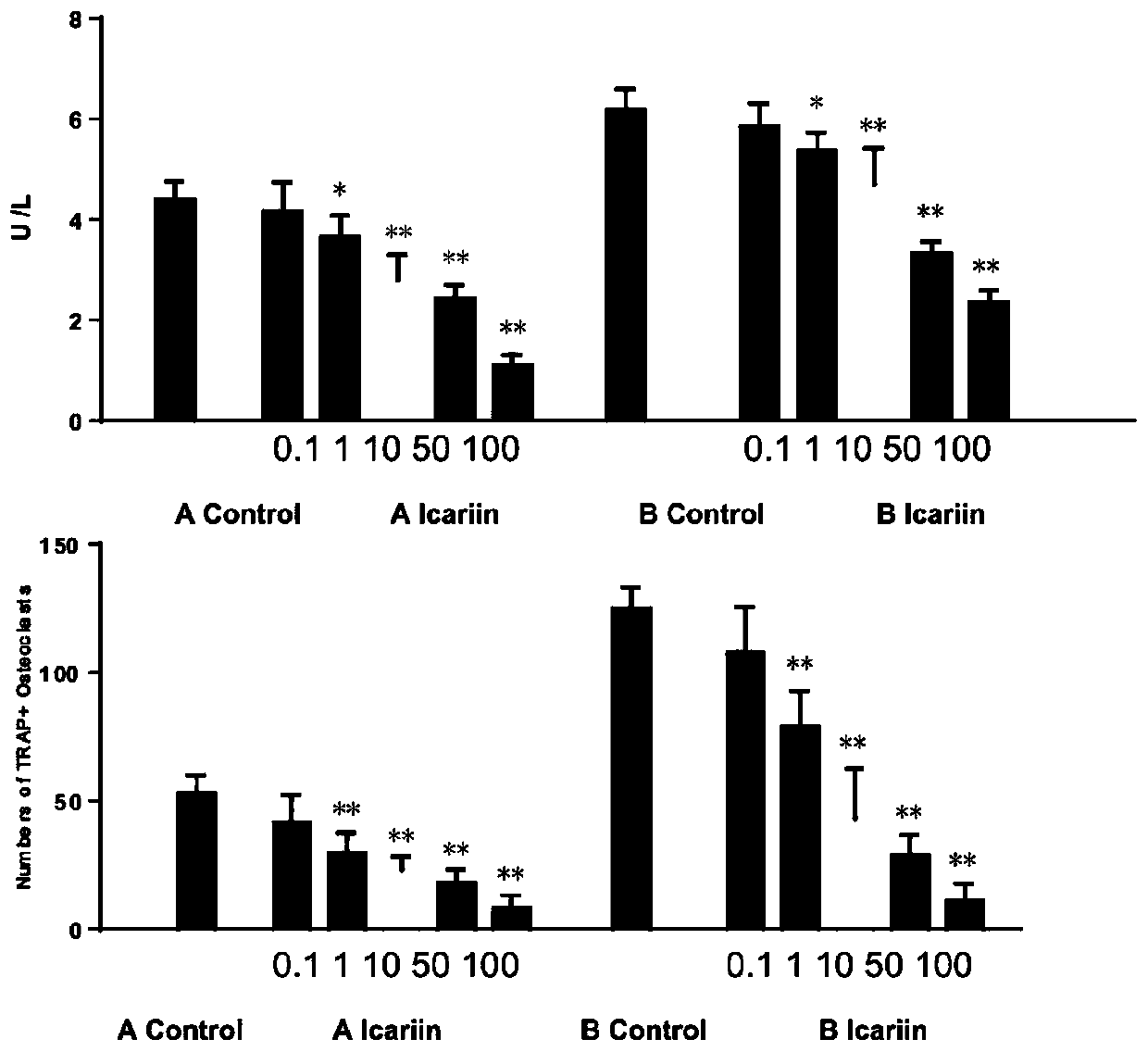
[0068] Use the microscope AXIO vert A1 type inverted microscope (ZEISS) to photograph (50 times) the cells in each well of the 96-well plate of embodiment 1 and embodiment 2, and take about 20 pictures in each well, and put the pictures together to form The overall picture of the whole hole, such as figure 1 shown. (Group A is the culture method of Example 2, and Group B is the culture method of Example 1) Image J software calculates the ratio of the osteoclast growth area to the total hole area, and the osteoclast growth area of group A accounts for 64.56% of the orifice plate area, Group B accounted for 88.48%, and there was a significant difference between Group B and Group A (P figure 2 shown. The osteoclasts cultured by the optimized culture method have a wider growth area on the well plate, as shown in the figure below, in the osteoclasts cultured at the same seed plate density, the number of osteoclasts is more and the growth is denser, showing Mosaic.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com