Medicine-lipiodol solvent and preparation method thereof
A technology of lipiodol and solvent, which is applied in the field of drug-iodol solvent and its preparation, and can solve problems such as unfavorable long-acting treatment, influence of lipiodol viscosity, unfavorable treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation of doxorubicin-lipiodol solvent includes the following steps:
[0065] (a) First, take 5 mg of doxorubicin, add it to 2 mL of ethanol, and use an ultrasonic cleaner to sonicate for 5 min to make it evenly mixed;
[0066] (b) then, in the autoclave, add 2mL lipiodol injection;
[0067] (c) then, add 200 μL of doxorubicin ethanol solution to the autoclave;
[0068] (d) After sealing the autoclave, pressurize it to 10Mpa through the system, and control the temperature at 30°C. After the temperature and pressure are stable, turn on the stirring paddle to stir at 9000 rpm, maintain the temperature and pressure, and stir for 1 hour;
[0069] (e) Then slowly depressurize and collect doxorubicin-lipiodol solvent.
Embodiment 2
[0071] The preparation of indocyanine green-lipiodol solvent comprises the following steps:
[0072] (a) First, weigh 1 mg of indocyanine green, add it to 2 mL of ethanol, and use an ultrasonic cleaner to sonicate for 5 min to make it evenly mixed;
[0073] (b) then, in the autoclave, add 5mL lipiodol injection;
[0074] (c) then, add 150 μL of doxorubicin ethanol solution to the autoclave;
[0075] (d) After sealing the autoclave, pressurize it to 20Mpa through the system, and control the temperature at 40°C. After the temperature and pressure are stable, turn on the stirring paddle and stir at 8000 rpm), maintain the temperature and pressure stability, and stir for 2h;
[0076] (e) The pressure was then slowly reduced, and the indocyanine green-lipiodol solvent was collected.
Embodiment 3
[0078] The preparation of indocyanine green / doxorubicin-lipiodol solvent includes the following steps:
[0079] (a) First, weigh 0.75 mg of indocyanine green, 0.75 mg of doxorubicin and add it to 2 mL of ethanol, and use an ultrasonic cleaner to sonicate for 5 min to make it evenly mixed;
[0080] (b) then, in the autoclave, add 2.5mL lipiodol injection;
[0081] (c) Then, add 150 μL of indocyanine green / doxorubicin ethanol solution to the autoclave;
[0082] (d) After sealing the autoclave, pressurize it to 15Mpa through the system, and control the temperature at 50°C. After the temperature and pressure are stabilized, turn on the stirring paddle to stir at 12,000 rpm, maintain the temperature and pressure, and stir for 0.5h;
[0083] (e) The pressure was then slowly reduced, and the indocyanine green / doxorubicin-lipiodol solvent was collected.
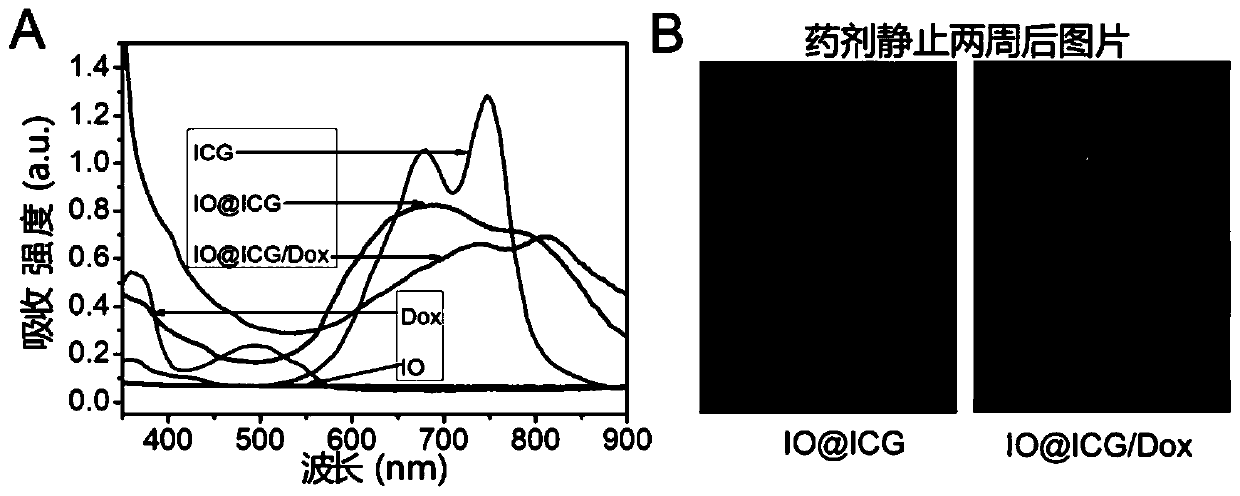
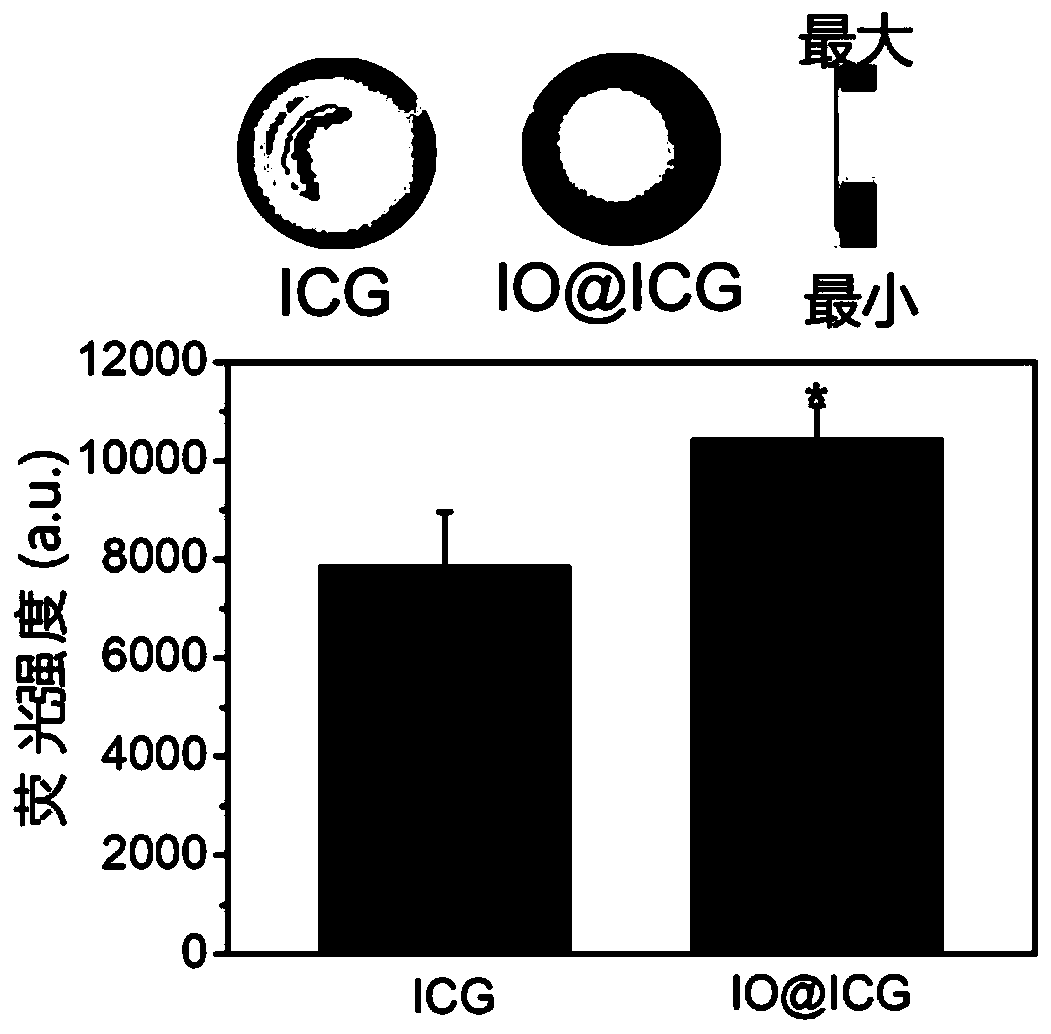
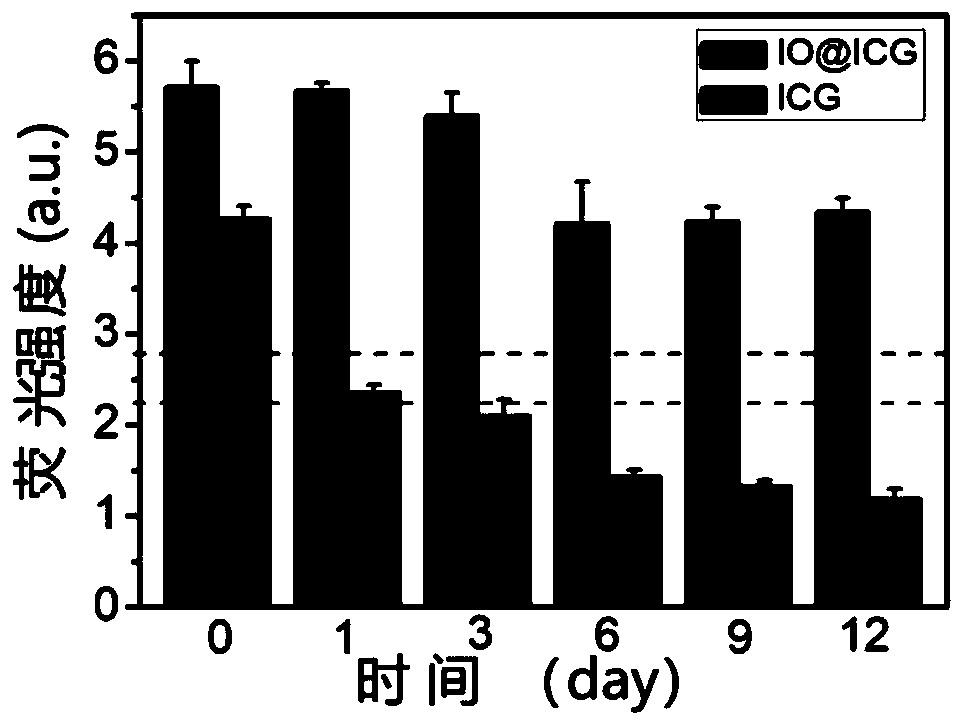
[0084] like figure 1 As shown, the absorption curve of ICG molecules in IO@ICG changed, but the characteristic peaks of ICG rema...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com