Method for preparing trifluoromethylsulfonyl fluoride from trifluoromethylsulfonyl chloride
A technology of trifluoromethanesulfonyl chloride and trifluoromethanesulfonyl, applied in the field of organic chemical industry, to achieve the effects of improving reaction speed and production efficiency, simple synthesis route, and high fault tolerance rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
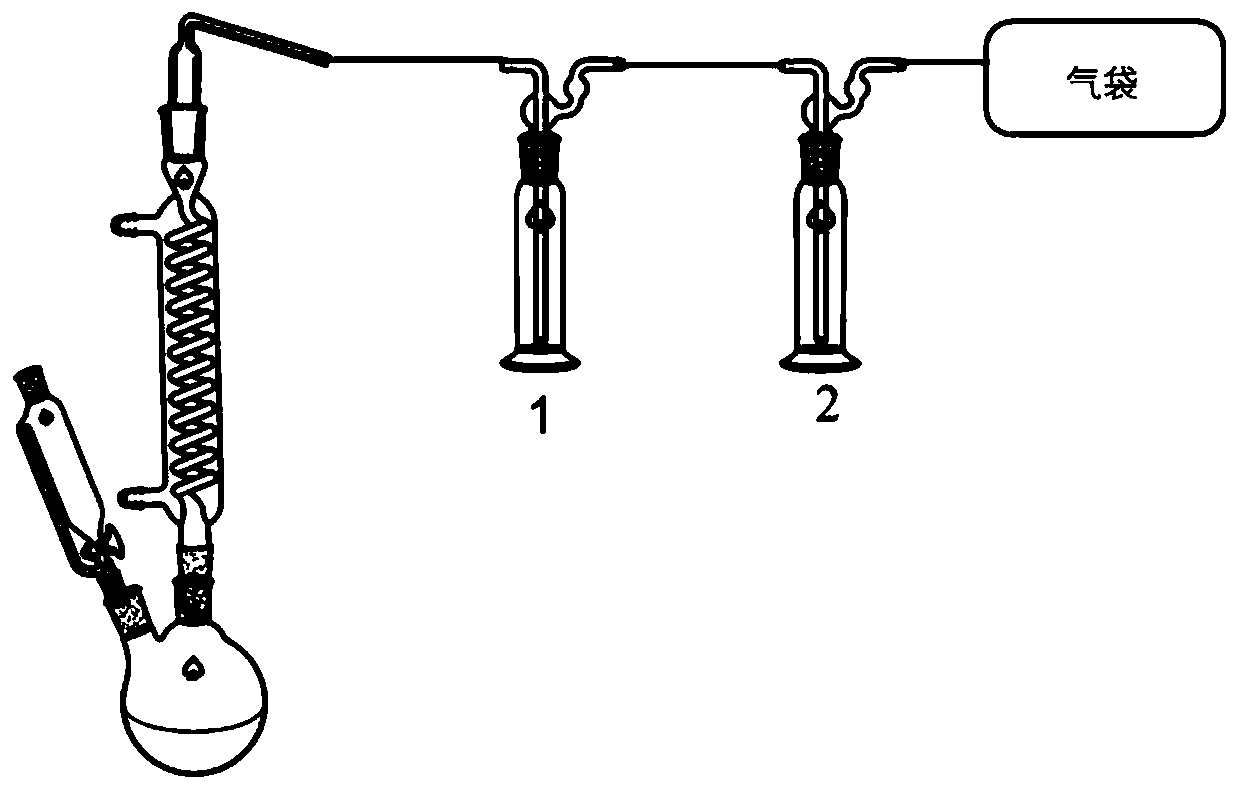
[0038] 10 g of potassium fluoride and 0.1 g of 18-crown-6 were added to 30 mL of anhydrous acetonitrile solution, 10 g of trifluoromethanesulfonyl chloride was added dropwise to the solution, and the solution was placed in an ice-water bath with the temperature kept at 0°C. After the dropwise addition was completed, it was kept for 2 hours. After the reaction was finished, 3.48 g of trifluoromethanesulfonyl fluoride could be collected in No. 2 cold trap, with a yield of 40% and a purity of 94.0%.
Embodiment 2
[0040] 100 g of potassium fluoride and 5 g of 18-crown-6 were added to 400 mL of anhydrous acetonitrile solution, 100 g of trifluoromethanesulfonyl chloride was added dropwise to the solution, and the solution was placed in an ice-water bath with the temperature kept at 0°C. After the dropwise addition was completed, it was kept for 2 hours. After the reaction was finished, 56.55 g of trifluoromethanesulfonyl fluoride could be collected in No. 2 cold trap, with a yield of 65% and a purity of 98.4%.
Embodiment 3
[0042] Add 50 g of potassium fluoride and 1.25 g of 18-crown-6 into 200 mL of anhydrous acetonitrile solution, add 50 g of trifluoromethanesulfonyl chloride dropwise into the solution, and place the solution in an ice-water bath with the temperature kept at 0°C. After the dropwise addition was completed, it was kept for 2 hours. After the reaction was finished, 28.00 g of trifluoromethanesulfonyl fluoride could be collected in No. 2 cold trap, with a yield of 64% and a purity of 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com