Lithium difluorobisoxalate phosphate preparation method, non-aqueous electrolyte and battery
A technology of difluorobis-oxalate lithium phosphate and bis-oxalate lithium phosphate, which is applied to non-aqueous electrolyte batteries, secondary batteries, lithium batteries, etc. , the product is difficult to purify and other problems, to achieve the effect of simple process, small internal resistance and electrochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
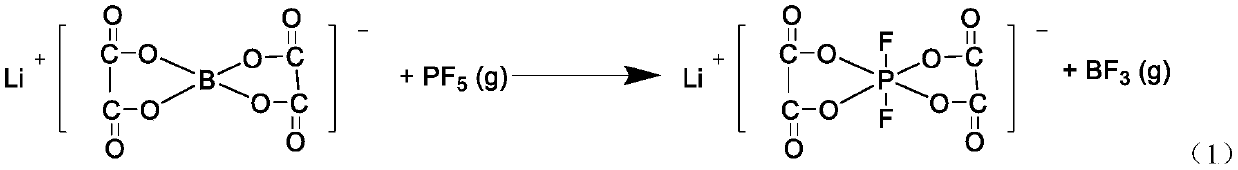
[0037] In a glove box, take 30 g of synthesized lithium bisoxalate borate with a moisture content below 200 ppm in a 500 mL three-necked flask, and add 309 mL of acetonitrile with a moisture content below 50 ppm dried over molecular sieves. Move it out of the glove box, immerse it in the set 60°C oil bath and stir it well, when the temperature in the flask is 60°C, pass into phosphorus pentafluoride gas (make the mixture of bisoxalate lithium borate and phosphorus pentafluoride The molar ratio is 1:1.05), and the total feeding time is controlled within 2-3 hours. After the gas feeding is completed, continue to stir for 2 hours to react. After the reaction is completed, remove insoluble impurities by filtration to obtain a lithium difluorobisoxalate phosphate solution. After vacuum distillation at 90-110°C, dichloromethane is added to the concentrated solution for crystallization to obtain a crude product of lithium difluorobisoxalate phosphate. Dry in a vacuum drying oven at a...
Embodiment 2
[0039]In the glove box, take 35.8 g of synthesized lithium bisoxalate borate with a moisture content below 200 ppm in a 250 mL three-necked flask, and add 185 ml of acetonitrile with a moisture content below 50 ppm dried with molecular sieves. Move it out of the glove box, immerse it in the set 80°C oil bath and stir it fully. When the temperature in the flask is 80°C, pass in phosphorus pentafluoride gas (make the mixture of bisoxalate lithium borate and phosphorus pentafluoride The molar ratio is 1:2), and the total feeding time is controlled at 2-3 hours. After the gas feeding is completed, continue to stir for 2 hours to react. After the reaction is completed, remove insoluble impurities by filtration to obtain a lithium difluorobisoxalate phosphate solution. After vacuum distillation at 90-110°C, dichloromethane is added to the concentrated solution for crystallization to obtain a crude product of lithium difluorobisoxalate phosphate. Dry in a vacuum drying oven at a temp...
Embodiment 3
[0041] In a glove box, take 36.5 g of synthesized lithium bisoxalate borate with a moisture content below 200 ppm in a 250 mL three-necked flask, and add 125 ml of tetrahydrofuran with a moisture content below 30 ppm dried with molecular sieves. Move it out of the glove box, immerse it in the set 80°C oil bath and stir it fully. When the temperature in the flask is 80°C, pass in phosphorus pentafluoride gas (make the mixture of bisoxalate lithium borate and phosphorus pentafluoride The molar ratio is 1:2.5), and the total feeding time is controlled at 2-3 hours. After the gas feeding is completed, continue to stir for 2 hours to react. After the reaction is completed, remove insoluble impurities by filtration to obtain a lithium difluorobisoxalate phosphate solution. After vacuum distillation at 90-110°C, dichloromethane is added to the concentrated solution for crystallization to obtain a crude product of lithium difluorobisoxalate phosphate. Dry in a vacuum drying oven at a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com