Naphthalimide-platinum (II) complex and preparation method and application thereof
A naphthalimide, the technology of the naphthalimide, which is applied in the field of naphthalimide-platinum complex and its preparation, can solve the problem of tumor cell restriction and the like, and achieves a simple synthetic route, good tumor suppressing effect, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
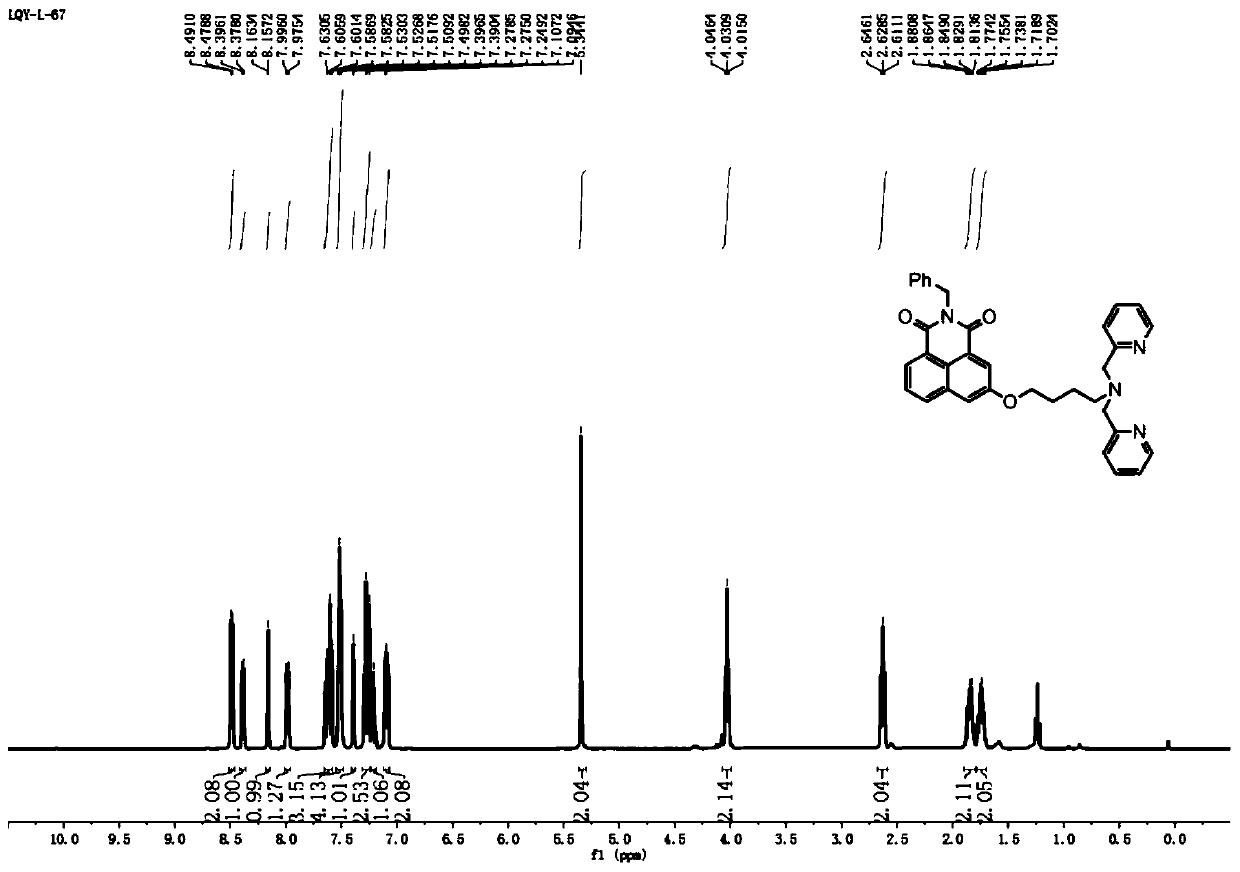
[0036] 1.1 Identification of the synthesized naphthalimide derivative ligand c
[0037] The proton nuclear magnetic resonance spectrum of the ligand c obtained is as follows figure 1 as shown, 1 H NMR (400MHz, CDCl3) δ8.48 (d, J = 4.9Hz, 2H), δ8.39 (d, J = 7.2Hz, 1H), δ8.16 (d, J = 2.5Hz, 1H), δ7 .99(d, J=8.2Hz, 1H), δ7.72-7.57(m, 3H), δ7.55-7.48(m, 4H), δ7.39(d, J=2.4Hz, 1H), δ7 .34-7.24(m, 2H), δ7.24-7.18(m, 1H), δ7.14-7.04(m, 2H), δ5.34(s, 2H), δ4.04-4.01(m, 2H ), δ2.63 (t, J=7.0Hz, 2H), δ1.93-1.80 (m, 2H), δ1.78-1.70 (m, 2H).
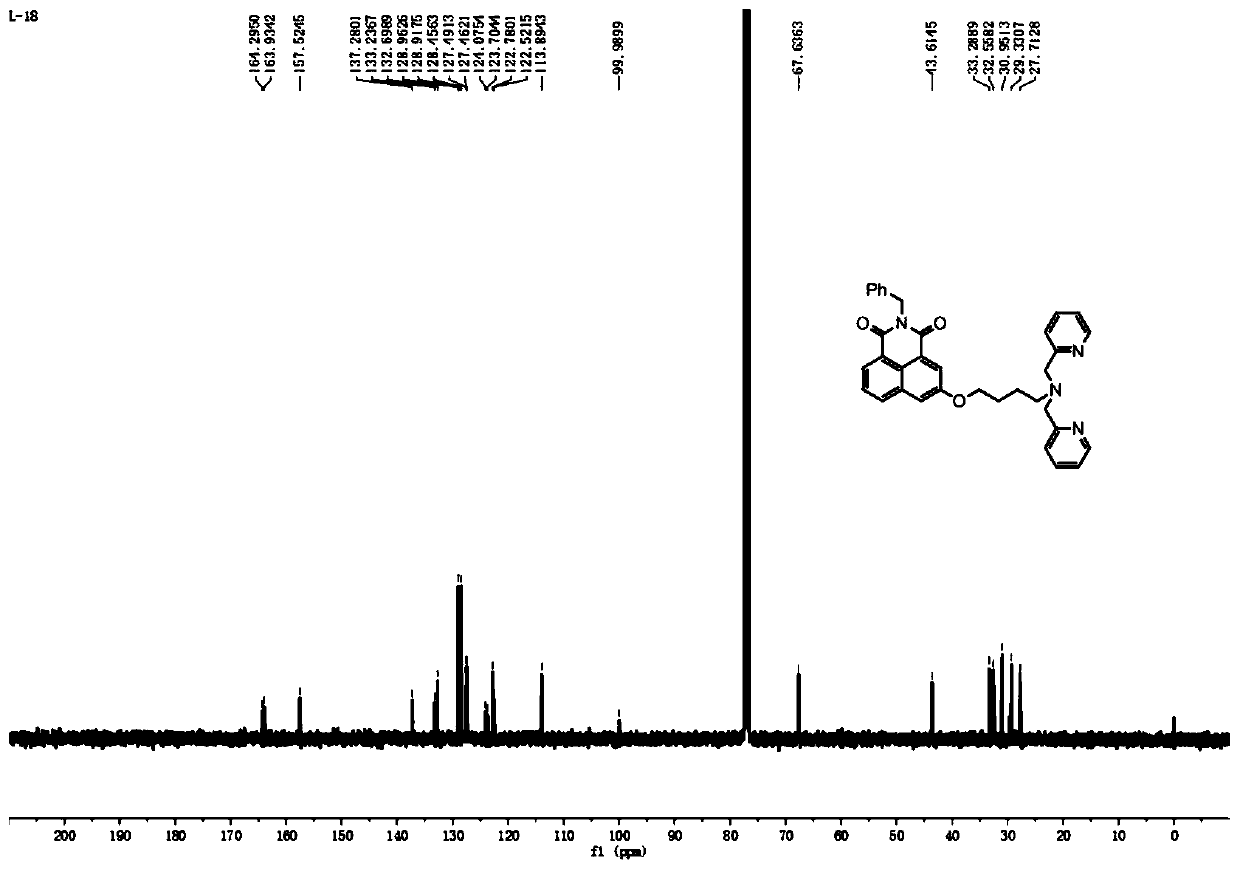
[0038] The carbon NMR spectrum of ligand c is as follows figure 2 as shown, 13 C NMR (101MHz, CDCl3) δ164.3, δ163.9, δ157.5, δ137.3, δ133.2, δ132.7, δ129.0, δ128.9, δ128.5, δ127.5, δ127.5 ,δ124.1,δ123.7,δ122.8,δ122.5,δ113.9,δ100.0,δ67.6,δ43.6,δ33.3,δ32.6,δ31.0,δ29.3,δ27 .7.
[0039] Therefore, can determine gained yellow ligand c, its structural formula is as follows:
[0040]
[0041] 1.2 Coordination reaction
[0042] Accurately weig...
Embodiment 2
[0059] In order to fully illustrate the use of the naphthalimide-platinum (II) complex described in the present invention in the preparation of antitumor drugs, antitumor activity experiments and toxicity experiments were carried out.
[0060] 1. Cell lines and cell culture
[0061] Five human cell lines were selected in this experiment: human cervical cancer cell HeLa, human lung cancer cell NCI-H460, human ovarian cancer SK-OV-3, human gastric cancer cell BEL-7402 and human normal liver cell HL-7702.
[0062] All human cell lines were cultured in RPMI-1640 medium containing 100U / mL penicillin, 10wt% calf blood, and 100U / mL streptomycin at 37°C with a volume concentration of 5% CO 2 cultured in an incubator.
[0063] 2. Preparation of test compounds
[0064] The purity of used ligand c and naphthalimide-platinum (II) complexes all need ≥ 95%, their DMSO stock solution is diluted into the final solution of 20 μ mol / L with physiological buffer (the final concentration of DMSO...
Embodiment 3
[0080] The complex inhibits the telomerase activity of NCI-H460 cells
[0081] 1. Cell Culture and Compound Formulation
[0082] The human ovarian cancer drug-resistant strain NCI-H460 is a tumor cell line, and the complex has an action time of 10.0 μM for 24 hours. The steps of cell culture and compound preparation are the same as in Example 2.
[0083] 2. Telomerase extraction and inhibition experiments
[0084] The telomerase extraction kit was purchased from Beijing Zhongxi Yuanda Company, item number NKJ15DLM, and stored at -80°C for a long time.
[0085] 2.1 Telomerase extraction
[0086] After the complex (10.0μM) acted on human lung cancer drug-resistant strain NCI-H460 cells (with a blank control group), the following experiments were carried out:
[0087] (1) Collect no less than 1×10 6 Cells (approximately the amount of cells in wells 1-2 of a 6-well plate), centrifuged at a speed of 2000rpm for 5min, collected the cells by centrifugation, washed with pre-cooled...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com