Roxatidine acetate pharmaceutical salt sustained-release pellets and preparation method and applications thereof
A technology of roxatidine acetate and sustained-release pellets, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, antibacterial drugs, etc., and can solve the problem of drug-loaded pellet coating compaction and drug-loaded layer coating Low drug loading and other problems, to achieve good stability and improve the effect of sustained release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
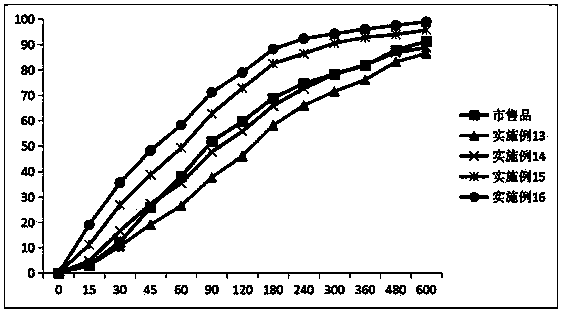
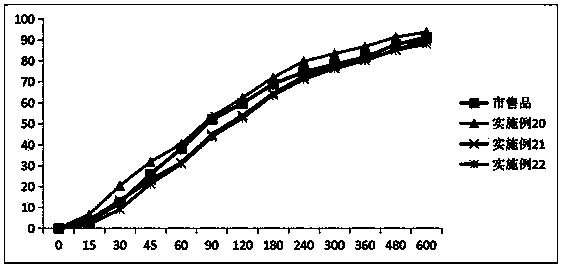
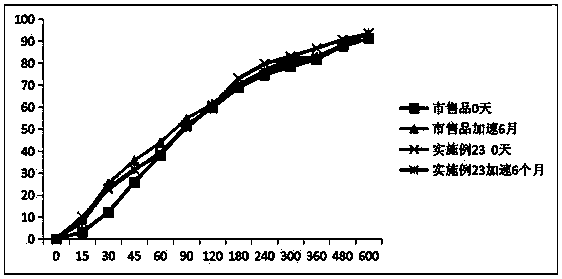
Image
Examples
Embodiment 1
[0057] Example 1 Preparation of Roxatidine Hydrochloride Acetate-loaded Pellets
[0058] Sucrose core 65mg
[0059] Roxatidine Acetate Hydrochloride 75mg
[0060] Hydroxypropyl Cellulose-SL 3.75mg
[0062] 95% ethanol 656.25mg
[0063] Mechanically pulverize the raw material, weigh 95% ethanol in the prescribed amount, add hydroxypropyl cellulose-SL and the main ingredient under stirring, add talcum powder after stirring evenly, stir, sieve, and set aside. Use a fluidized bed granulation coating machine to spray the drug-loaded coating solution onto the blank pellet core. The fluidized bed coating conditions are: air intake air volume: 120-160m 3 / h, air inlet temperature: 30-50°C, atomization pressure 1.6-2.2kg / cm 2 . During the coating process, the coating pellets were cohesive and hardened, and the coating could not be completed and the theoretical weight gain could not be achieved.
Embodiment 2
[0064] Example 2 Preparation of Roxatidine Hydrochloride Acetate-loaded Pellets
[0065] Sucrose core 65mg
[0066] Roxatidine Acetate Hydrochloride 75mg
[0067] Hydroxypropyl Cellulose-SL 3.75mg
[0069] Absolute ethanol 656.25mg
[0070] Mechanically pulverize the raw materials, weigh the prescribed amount of absolute ethanol, add hydroxypropyl cellulose-SL under stirring, stir until dissolved, add the main ingredient, stir evenly, then add talcum powder, stir evenly, sieve, and set aside . Spray the drug-loaded coating solution onto the blank pellet core with a fluidized bed granulator, dry and sieve to obtain the drug-loaded pellets. The coating conditions are the same as in Example 1, the absolute ethanol is chemically pure or above, and the weight fraction of ethanol alcohol is greater than 99.5%.
Embodiment 3
[0071] Example 3 Roxatidine Hydrochloride Acetate Loaded Pellets
[0072] Sucrose core 65mg
[0073] Roxatidine Acetate Hydrochloride 75mg
[0074] Hydroxypropyl Cellulose-L 3.75mg
[0075] Talc powder 15mg
[0076] Absolute ethanol 656.25mg
[0077] Mechanically pulverize the raw materials, weigh the prescribed amount of absolute ethanol, add hydroxypropyl cellulose-L while stirring, stir until dissolved, add the main ingredient, stir evenly, then add talcum powder, stir evenly, sieve, and set aside . Using a fluidized bed granulation coating machine, using the same conditions as in Example 2, the drug-loaded coating solution was sprayed onto the blank core, dried and sieved to obtain drug-loaded pellets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com