Method for preparing aldehyde by hydroformylation of olefins
A technology for the hydroformylation of olefins, applied in the preparation of carbon monoxide reactions, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of reduced activity and selectivity of supported catalysts, metal utilization The efficiency is not high, to achieve the effect of good selectivity of the target product, good catalyst stability and high catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
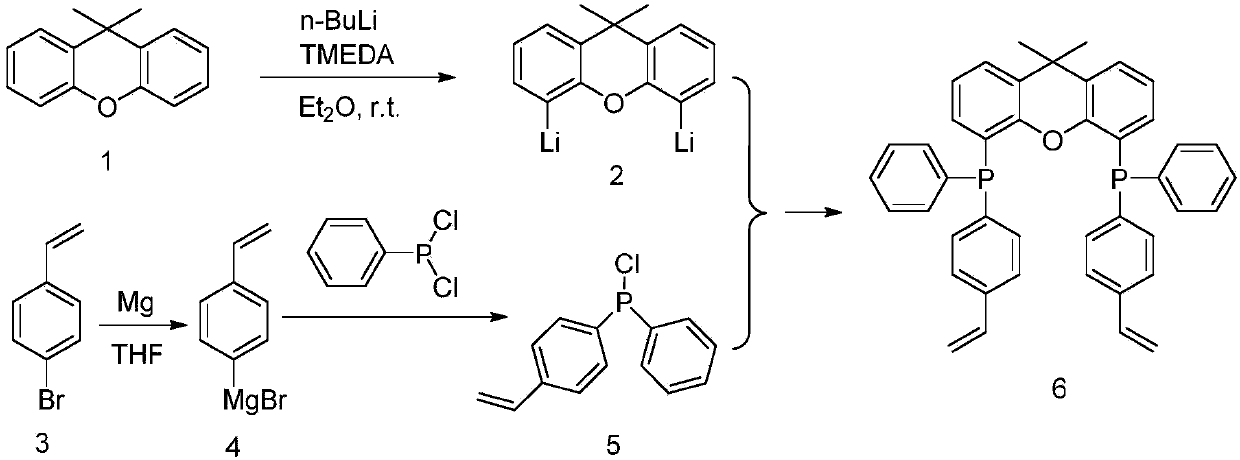
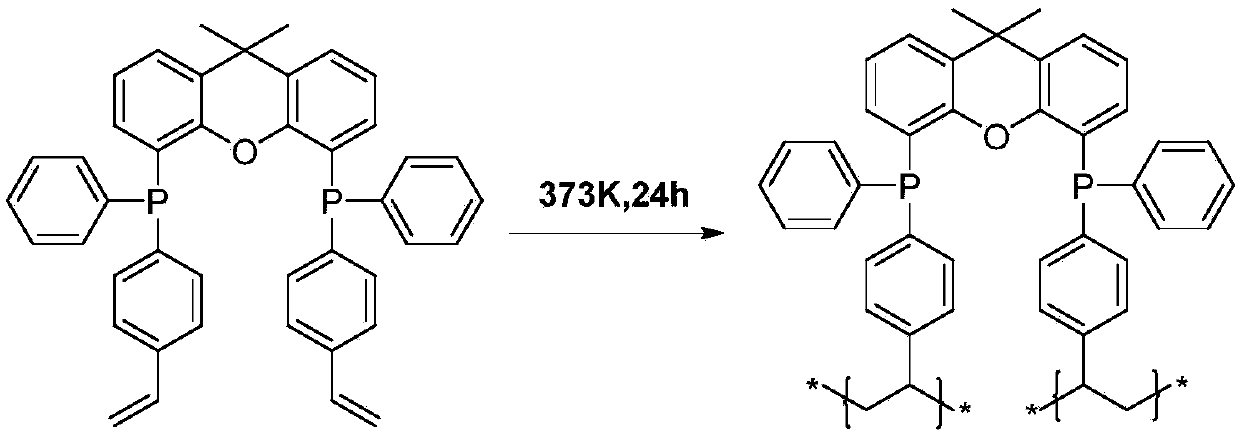
[0043] Preparation of the diphosphine ligand 2v-Xantphos: The synthetic route of the 2v-Xantphos ligand is attached figure 1 shown. Since 9,9-dimethylxanthene (with figure 1 Compound No. 1) has heteroatom O at the β-positions of the 4 and 5 positions, so it is easier to undergo deprotonated lithiation to generate dilithium reagent 2. Compound 5 was obtained by reacting p-bromostyrene 3 with phenylphosphine chloride after activation by Grignard reagent. The 2v-Xantphos ligand can be obtained by reacting compound 5 with dilithium reagent 2 at a molar ratio of 2:1. According to the calculation of raw material 1, the reaction yield is about 60%, and no purification and separation steps are required in the intermediate preparation process. The finally obtained 2v-Xantphos ligand is recrystallized from acetonitrile to obtain a white powdery solid. attached Figure 4 And attached Figure 5 NMR for the prepared 2v-Xantphos ligand 1 H and 31 P Spectrum.
[0044] Preparation of ...
Embodiment 2
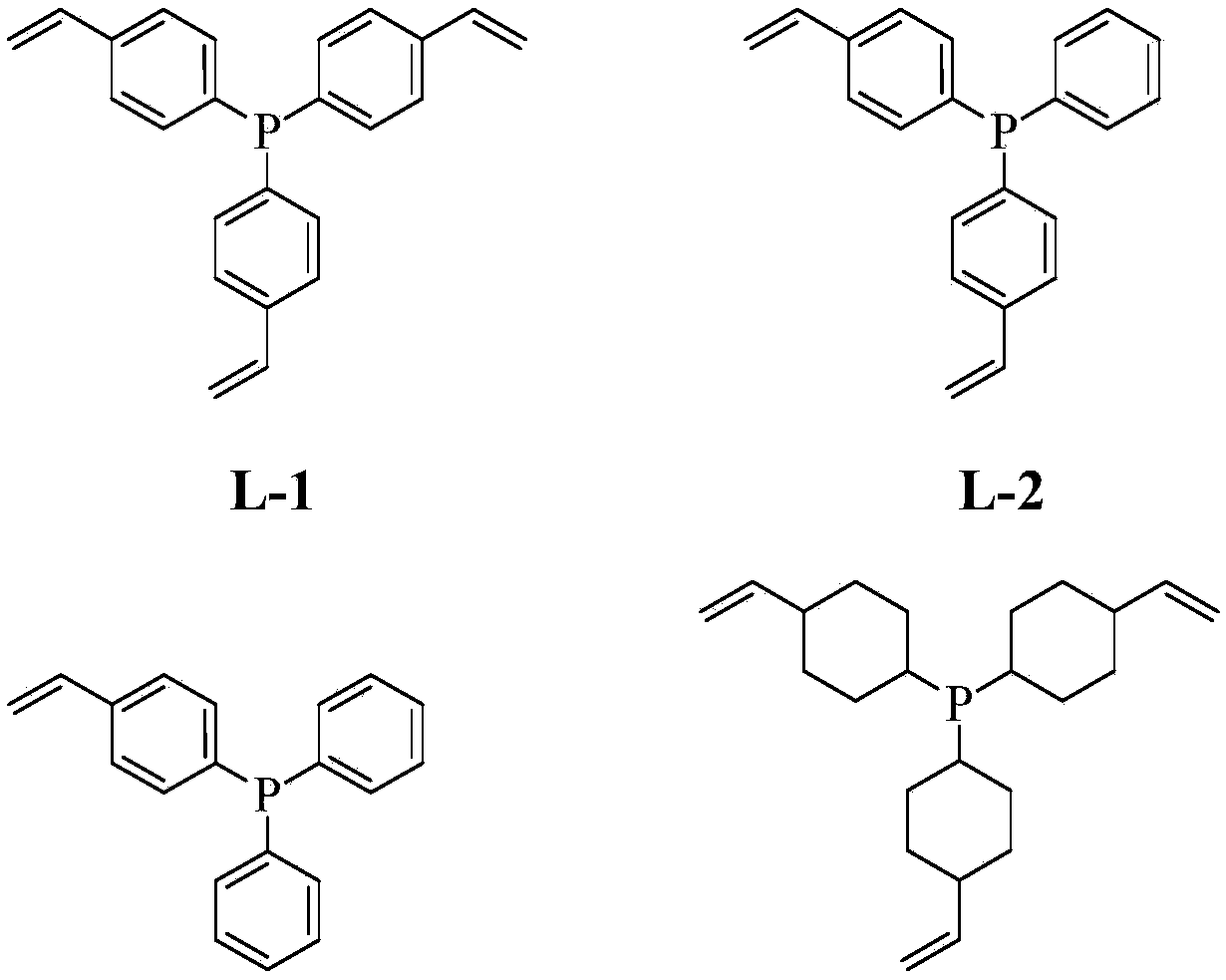
[0047] In Example 2, except taking 10.0 grams of comonomer tris (4-vinylphenyl) phosphine (L1), replacing 2.5 grams of comonomer tris (4-vinylphenyl) phosphine, the rest of the catalyst synthesis process Same as Example 1.
Embodiment 3
[0049] In Example 3, except that 0.01 gram of free radical initiator azobisisobutyronitrile was weighed instead of 1.0 gram of free radical initiator azobisisobutyronitrile, the rest of the catalyst preparation process was the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com