A pentacene derivative, its preparation method and its use in the detection of polyamines
A derivative, pentastyrene technology, applied in the field of compound detection, can solve the problems of insufficient detection sensitivity of fluorescent probes and small degree of fluorescence change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 - the synthesis of formula I compound
[0059] In this embodiment, a representative compound of formula I in which n is 3 and R is H is synthesized, represented by the code SSS1.
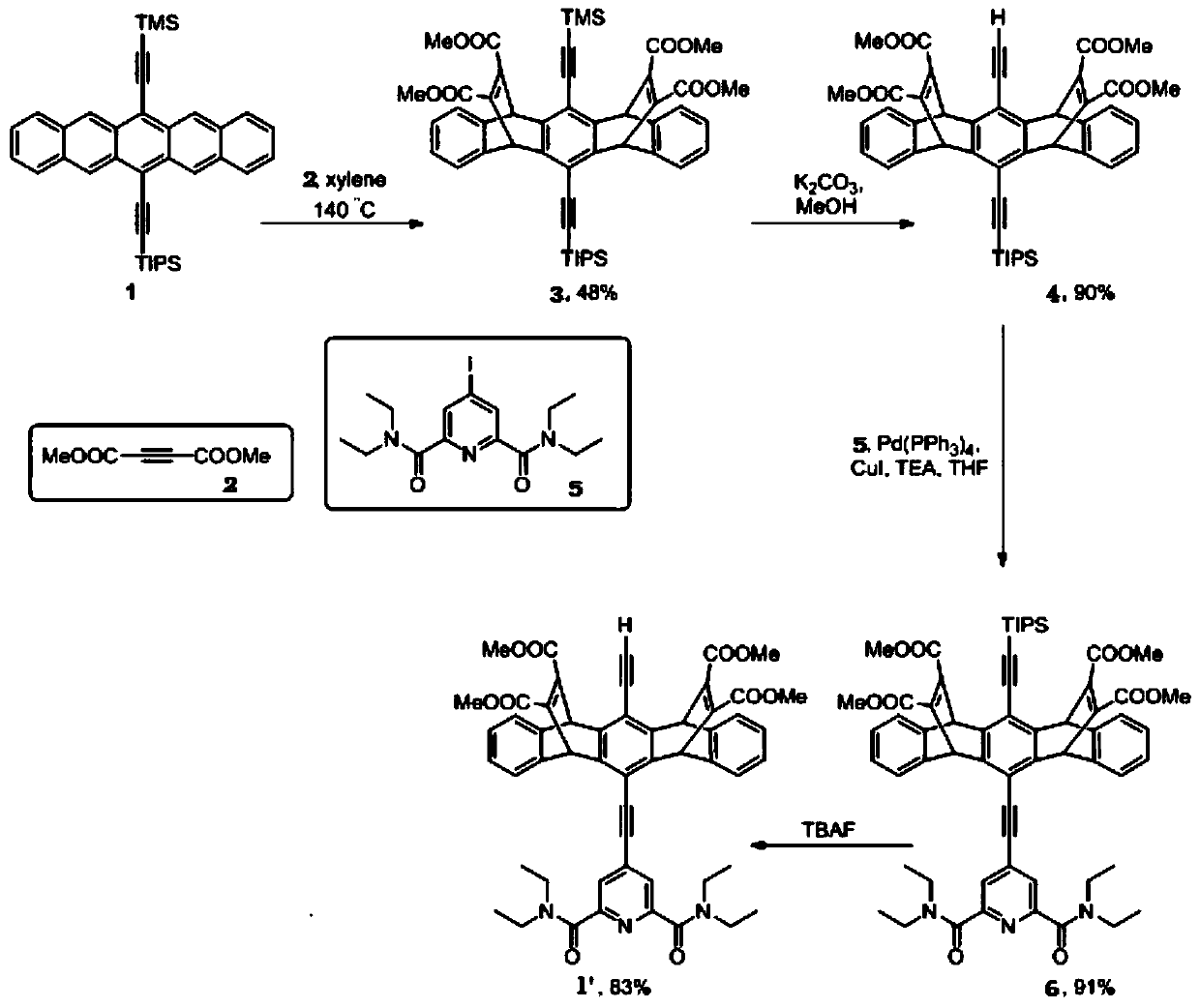
[0060] The synthetic route of the formula I compound SSS1 of the present embodiment is as follows figure 1 and figure 2 shown. Except for the starting compound 1 shown in the following formula 1, other compounds used in the synthesis are commercially available. The synthetic method of starting compound 1 shown in following formula 1 is in literature Satrijo A., Kooi S., Swager T., Enhanced Luminescence from Emissive Defects in Aggregated Conjugated Polymers, Macromolecules 2007,40,8833-8841 and LehnherrD., McDonald R ., reported in Tykwinski R. Exploring Electronically Polarized Pentacenes Org. Lett. 2008, 10, 4163-4166, the relevant contents of these two documents are incorporated herein by reference.
[0061] (1) Synthesis of intermediate compound 1'
[0062] figure 1 It...
Embodiment 2
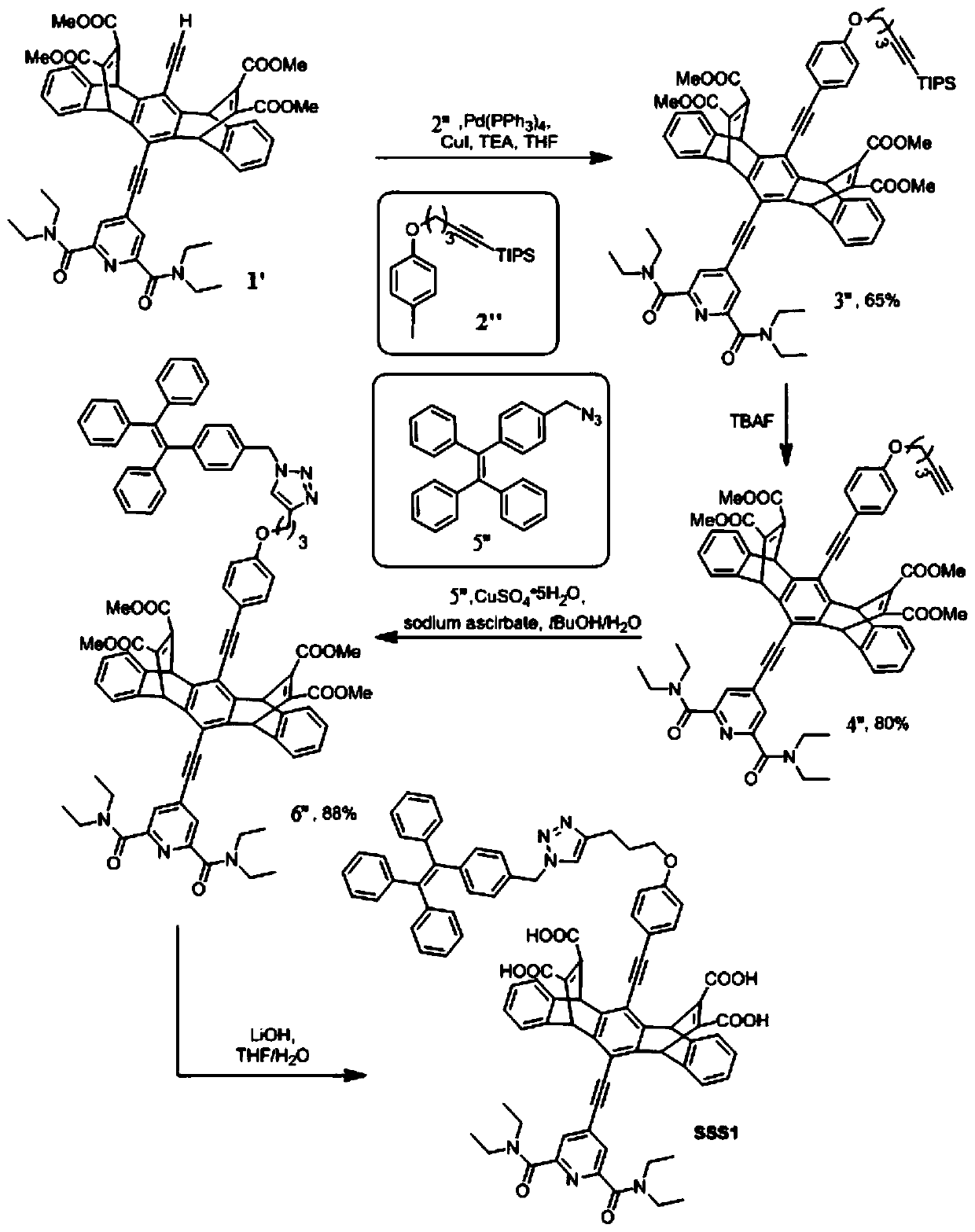
[0088] Embodiment 2 - the synthesis of formula I compound
[0089] This embodiment synthesizes other representative compounds of formula I, respectively represented by codes SSS2, SSS3 and SSS4, wherein respectively, n is 1, R is H, or n is 1, R is OMe, or n is 3, R for OMe. The method of synthesis is analogous to Example 1, with the exception that a compound of formula 2" with a corresponding value of n and a compound of formula 5" with a corresponding R group are used. The structural identification data of the final product are listed below:
[0090] SSS2: n=1, R=H; pale yellow powdery solid (33.6mg, 0.024mmol, 80%), m.p.>300°C. 1 HNMR(400MHz,MeOD)δ8.00(s,2H),7.85(s,1H),7.73–7.61(m,2H),7.36–7.19(m,4H),7.09–6.97(m,14H),6.97 –6.84(m,7H),6.84–6.70(m,4H),5.92(s,4H),5.40(s,2H),5.13(s,2H),3.56(q,J=7.0Hz,4H), 3.35 (q, J=7.0Hz, 4H), 1.33–1.21 (m, 6H), 1.21–1.07 (m, 6H). 13 CNMR (100MHz, MeOD) δ169.0, 168.1, 160.0, 158.8, 154.0, 147.3, 146.0, 145.4, 145.3, 145.2, 144.4, 144.1, 1...
Embodiment 3
[0093] Embodiment 3 - the characterization of formula I compound SSS1
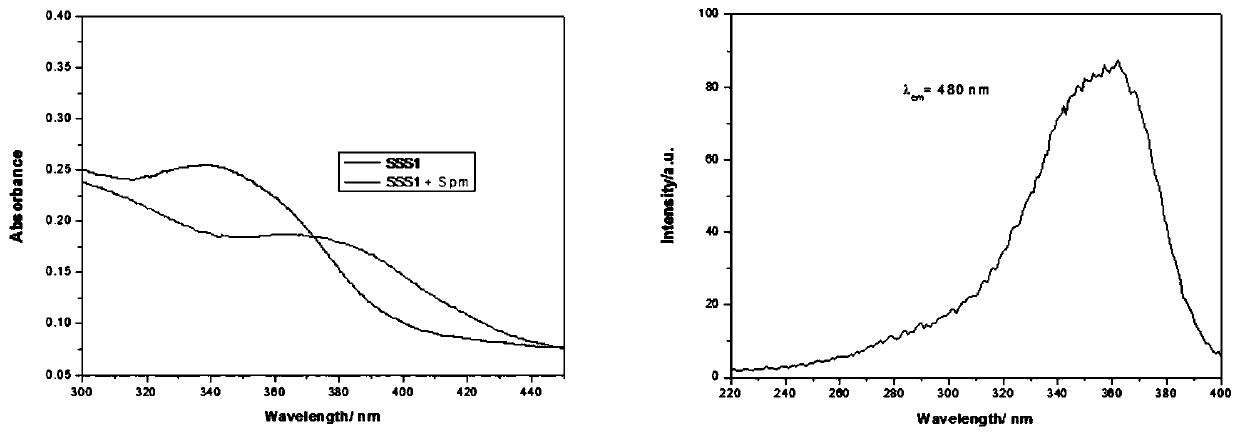
[0094] (1) The photophysical spectrogram of formula I compound SSS1
[0095] SSS1 was dissolved in 1% methanol / water solution to prepare a solution with a concentration of 10 μM. Measure its UV-Vis absorption spectrum with a UV-Vis spectrophotometer (Shimadzu Corporation UV-2600 type), the results are as follows: image 3 shown in the left figure. The two curves in the left figure are the ultraviolet absorption spectra of the SSS-1 solution alone and the SSS-1 solution after adding 1 equivalent of Spm. After adding Spm, there is an obvious red shift, indicating that there is an interaction between Spm and SSS-1. Use this solution in fluorescence spectrometer (Japan Shimadzu RF-5301), take 480nm as emission wavelength, measure the excitation spectrum of SSS1, the result is as follows image 3 shown in the figure on the right. It can be seen from the figure on the right that under the excitation waveleng...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com