Lanthanum-iron modified zeolite phosphorus removal adsorbent and preparation method and application thereof
A kind of technology of modifying zeolite and adsorbent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation method of phosphorus removal adsorbent, its step is as follows:
[0060] 1) Dissolve 0.008mol of lanthanum nitrate hexahydrate and iron nitrate nonahydrate in 200mL of deionized water at a molar ratio of 1:2, and mix well to form a mixed solution of lanthanum salt and iron salt;
[0061] 2) Add 10 g of zeolite to the mixed solution of lanthanum salt and iron salt in step 1), mix well, and stir for 3 hours to make an intermediate reaction solution;
[0062] 3) Evenly and slowly add sodium hydroxide solution to adjust the pH of the intermediate reaction solution obtained in step 2) to 9.5. After continuing to stir for 3 hours, turn off the magnetic stirrer, leave the intermediate reaction solution to age for 24 hours, and use a centrifuge to achieve solid-liquid separation of the solution , The centrifuge speed is 3500rpm. Wash the precipitate with water several times until the supernatant reaches a neutral pH of 7, and collect the precipitate;
[0063] ...
Embodiment 2
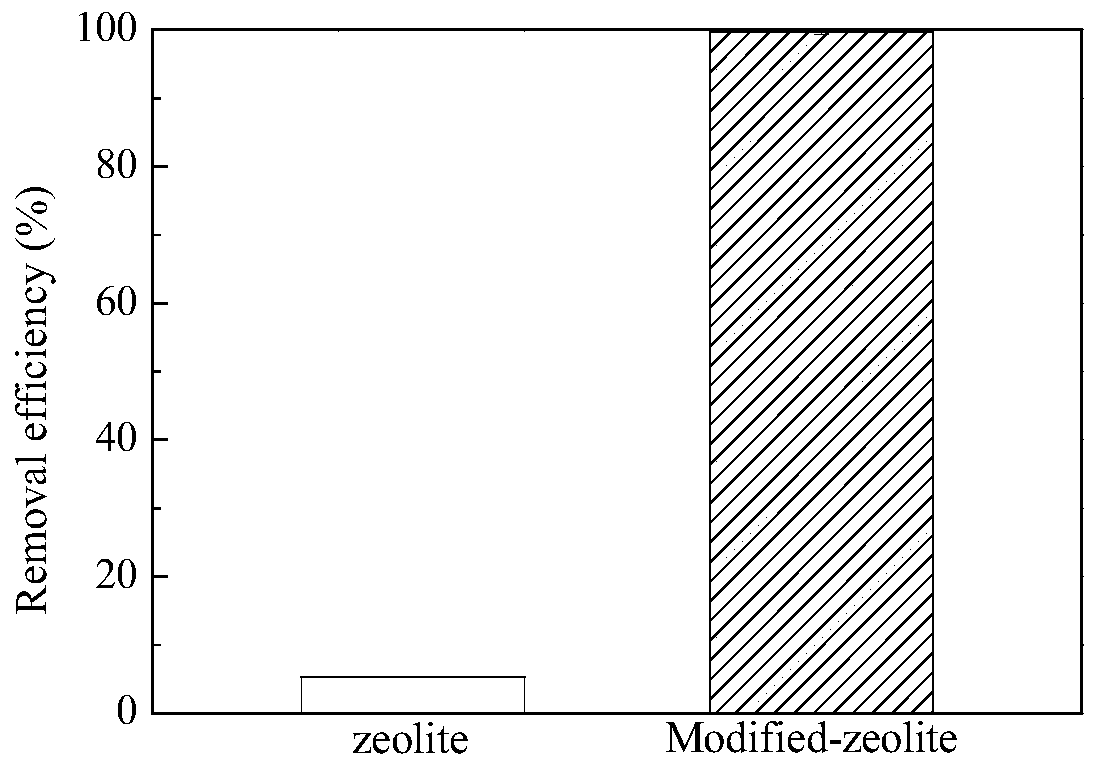
[0065] In order to explore the phosphorus removal effect after adsorbent modification, this embodiment compares the phosphorus removal performance of natural zeolite before and after modification (such as figure 1 )
[0066] The method for preparing adsorbent is the same as in Example 1.
[0067] The experimental process of adsorbent phosphorus removal effect is as follows: the phosphate solution of preparation 2mg / L is placed in the beaker, adds the zeolite before the modification of 0.5g / L and the adsorbent prepared in Example 1 respectively (dosage is determined by previous experiment ), fully reacted for 4h, measured the content of phosphate in the solution, and calculated and compared the removal rates of phosphate of the two kinds of adsorbents. In the modified zeolite experimental group, the phosphate removal rate increased from 5.34% to 99.66% in 4 hours, and the phosphorus removal effect of the adsorbent was significantly improved. After the reaction, the phosphate c...
Embodiment 3
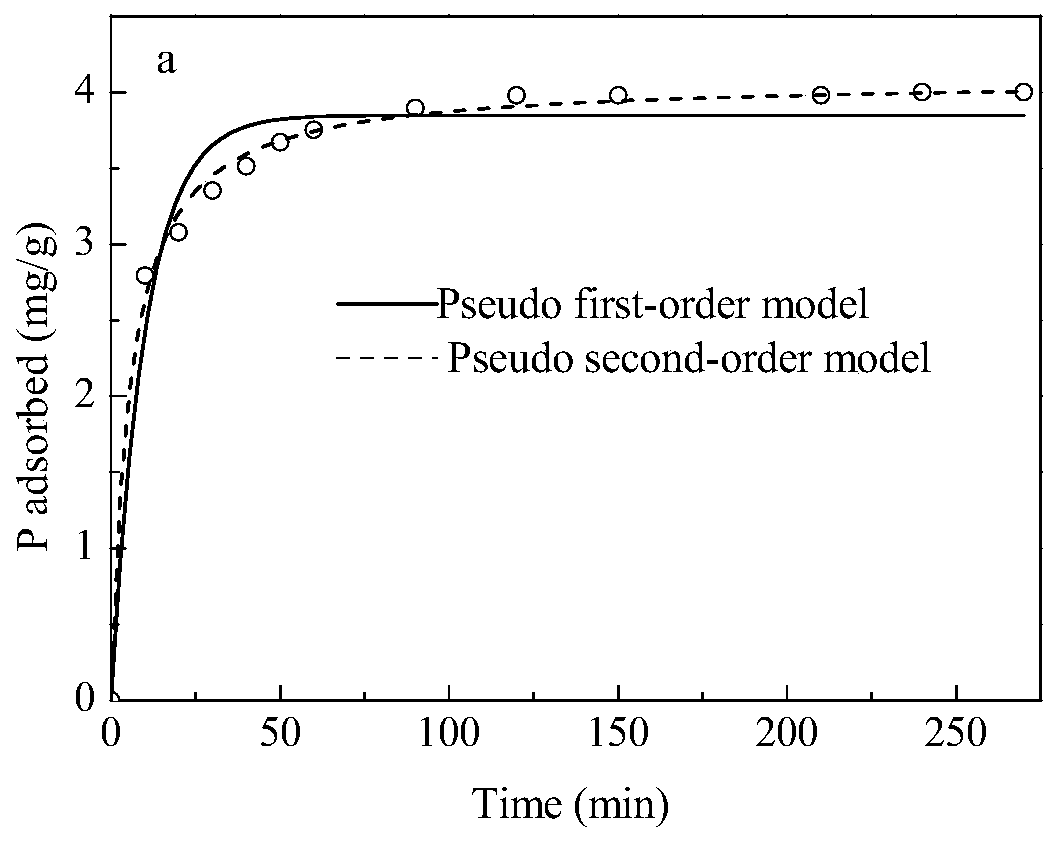
[0071] In order to explore the influence of adsorbent and phosphate contact time on adsorbent dephosphorization, this embodiment measures the concentration of phosphate in the solution at different times, and obtains the kinetic curve of adsorbent adsorption of phosphate (such as figure 2 ).
[0072] The method for preparing adsorbent is the same as in Example 1.
[0073] The adsorption kinetics experiment process is as follows: configure 2 mg / L phosphate solution and place it in a beaker, add 0.5 g / L adsorbent, start adsorption and record the reaction time, respectively at 10, 20, 30, 40, 50, 60, Samples were taken at 90, 120, 150, 210, and 270 minutes (3 parallels) to measure the phosphate concentration. The scatter diagram of adsorbent adsorption amount and contact time was drawn by software, and the experimental results were fitted by adsorption pseudo-first-order kinetic equation and pseudo-second-order kinetic equation, respectively. It can be found that the adsorptio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com