Detection method for opioid active substances based on cell dopamine release effect and detection kit for opioid active substance
A technology for detection kits and active substances, which is applied in the field of detection methods and detection kits for opioid active substances, can solve the problems of low component content, undetectable, impossible immunoassay, etc., and achieve rapid detection, high The effect of sensitive detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Construction of pCMV-MOR1 plasmid
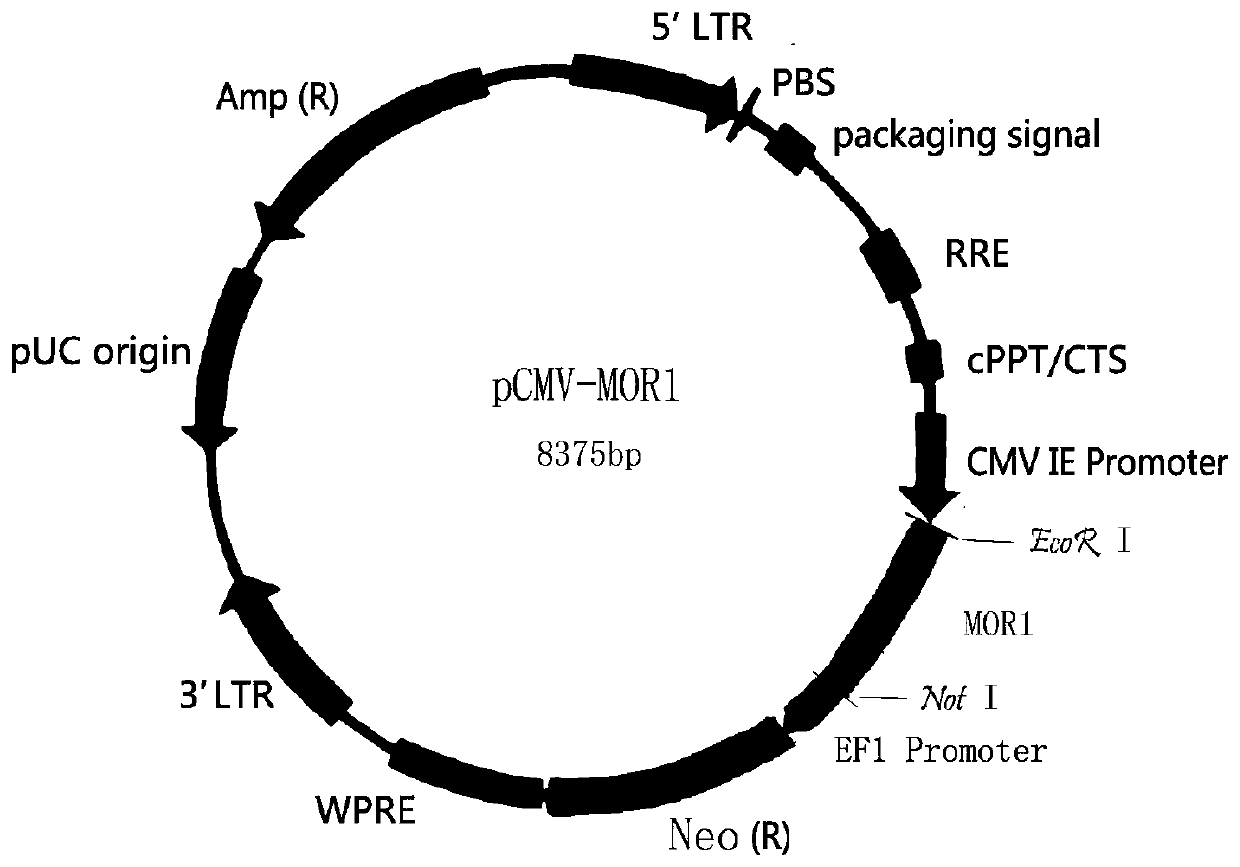
[0037] Ligase site EcoR I and not 1, the human mu type opioid receptor MOR1 gene is cloned under the CMV promoter of the lentiviral expression vector pCDH-CMV-MCS-EF1-Neo, and the eukaryotic expression plasmid pCMV-MOR1 (such as figure 1 shown).
Embodiment 2
[0038] Example 2 Establishment of MOR1 / SK-N-SH stable cell line and blank control Neo / SK-N-SH stable cell line
[0039] The pCMV-MOR1 plasmid, pH1 plasmid, and pH2 plasmid were co-transfected into lentiviral packaging line cell 293V to prepare MOR1 lentivirus, and transfected into SK-N-SH cells. G418 screening and cloning established MOR1 / SK-N-SH stable cells. transfected cell lines. Co-transfect the pCDH-CMV-MCS-EF1-Neo plasmid, pH1 plasmid, and pH2 plasmid into the lentiviral packaging line cell 293V to prepare empty vector lentivirus, and transfect SK-N-SH cells, G418 screening, clone establishment blank Control Neo / SK-N-SH stably transfected cell line. Specific steps are as follows:
[0040] 1) Preparation of packaging line cells: One day before transfection, use DMEM-H complete culture medium (containing 10% FBS and 100U / ml penicillin, 100μg / ml streptomycin double antibody) to make lentiviral packaging line cells 293V into 1 ×10 6 Inoculate a D19cm cell culture dish a...
Embodiment 3
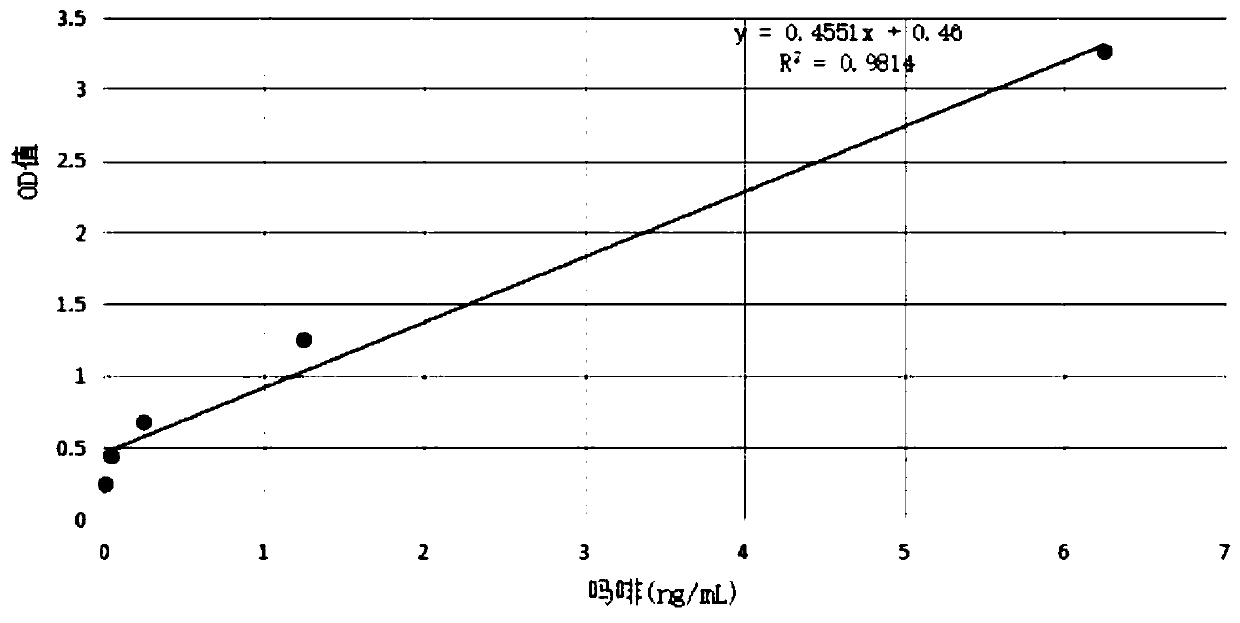
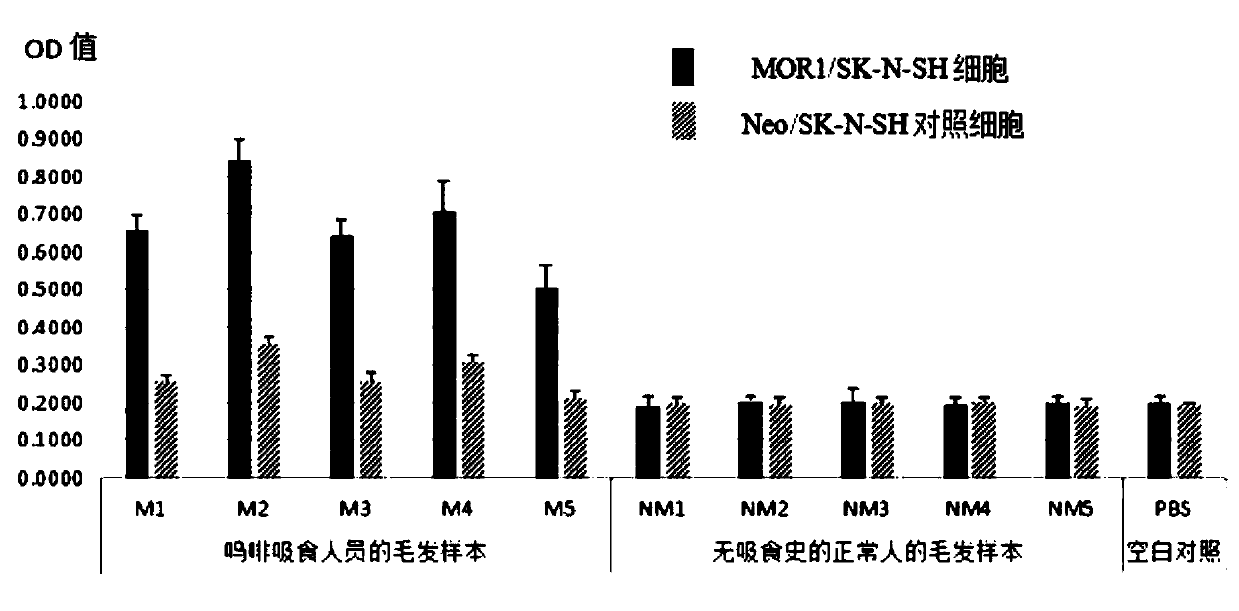
[0049] Example 3 Application of Universal Detection Kit for Opioid Active Substances
[0050] The universal detection kit for opioid active substances developed based on the technical solution of the present invention can be applied to the detection of various samples containing opioid active substances. In this embodiment, we take the hair of a person taking morphine as an example. Specific steps are as follows:
[0051] 1) Hair sample processing: Take 5 hair samples of morphine addicts and 5 hair samples of normal people without smoking history, and the numbers are shown in Table 1.
[0052]
[0053] Cut the hair sample within 3cm of the hair root at 20mg / part, cut it into pieces, put it into a 5ml EP tube, add 2ml of HBSS buffer solution (pH7.4) containing 1% keratinase, add a small amount of zirconium beads and quartz sand, and crush it Shake and pulverize for 1 minute to obtain corresponding sample liquids.
[0054] 2) Cell preparation: mix 5×10 cells one day in adv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com