A waste gas treatment method for a reaction process of preparing epoxy chloropropane from chloropropene
An epichlorohydrin and chloropropene technology, applied in the disproportionation separation/purification of halogenated hydrocarbons, organic chemistry, etc., can solve the problems of chloropropene loss, air pollution, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
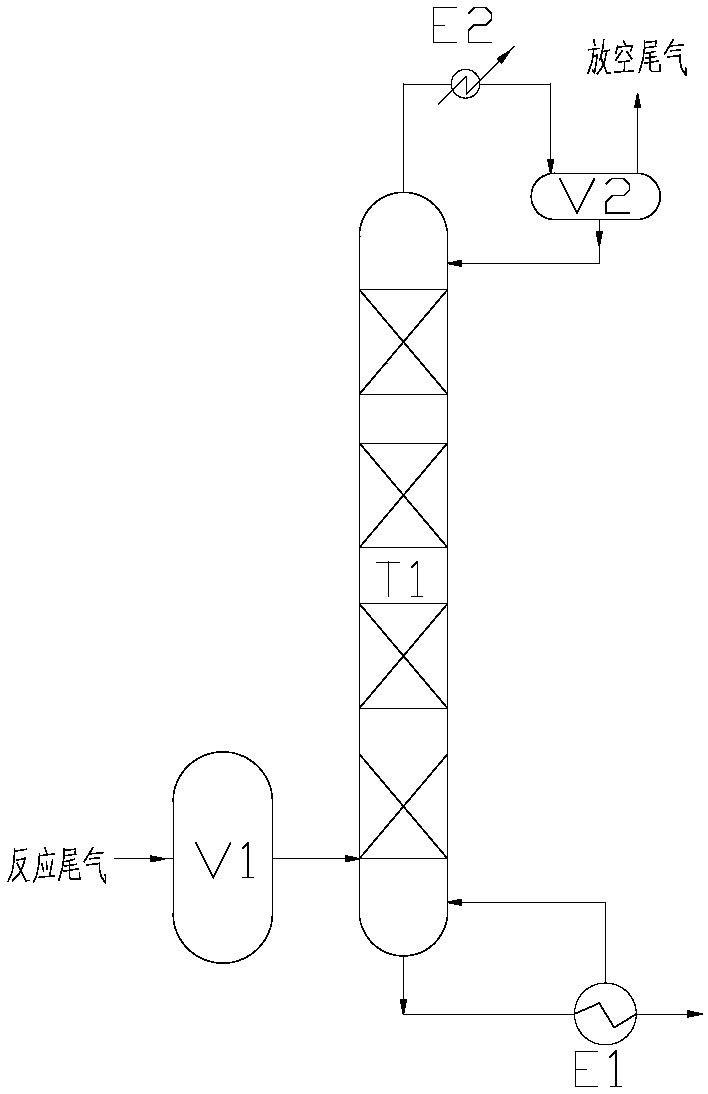
[0018] Set the temperature of the tower bottom heat exchanger E1 to 10°C;
[0019] Set the temperature of the top condenser E2 heat exchanger to -15°C;
[0020] Set the pressure range of absorption tower T1 as: 100kPa,
[0021] The filling amount of epichlorohydrin in the tower bottom of the absorption tower T1 is set to be 50% of the volume of the tower bottom.
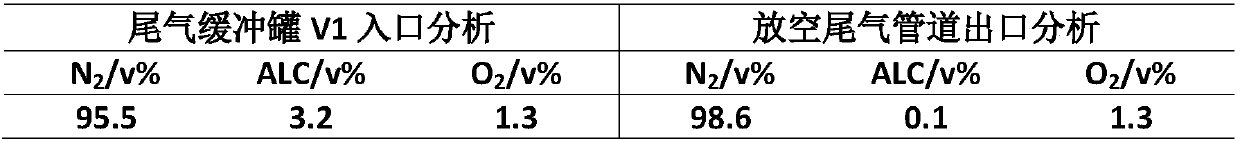
[0022] Using hydrogen peroxide as the oxygen source, the reaction-controlled phase transfer catalyst catalyzes the epoxidation of chloropropene to prepare epichlorohydrin. The results of waste gas treatment in the reaction process are shown in Table 1. It can be seen that the organic matter in the waste gas after this process is greatly reduced, reducing the The content of allyl chloride in the exhaust gas.
[0023] Table 1. Waste gas treatment results of the epoxidation of propylene chloride to prepare epichlorohydrin
[0024]
Embodiment 2
[0026] Set the temperature of the tower bottom heat exchanger E1 to 0°C;
[0027] Set the temperature of the top condenser E2 heat exchanger to -20°C;
[0028] Set the pressure range of absorption tower T1 as: 500kPa,
[0029] The filling amount of epichlorohydrin in the tower bottom of the absorption tower T1 is set to be 65% of the volume of the tower bottom.
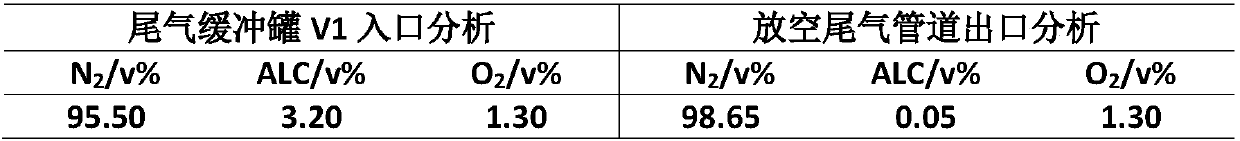
[0030] Using hydrogen peroxide as the oxygen source, the reaction-controlled phase transfer catalyst catalyzes the epoxidation of chloropropene to prepare epichlorohydrin. The results of waste gas treatment in the reaction process are shown in Table 2. It can be seen that the organic matter in the waste gas after this process is greatly reduced, reducing the The content of allyl chloride in the exhaust gas.
[0031] Table 2. Waste gas treatment results of the epoxidation of propylene chloride to prepare epichlorohydrin
[0032]
Embodiment 3
[0034] Set the temperature of the tower bottom heat exchanger E1 to 15°C;
[0035] Set the temperature of the top condenser E2 heat exchanger to -10°C;
[0036] Set the pressure range of absorption tower T1 as: 300kPa,
[0037] The filling amount of epichlorohydrin in the tower bottom of the absorption tower T1 is set to be 45% of the volume of the tower bottom.
[0038] Using hydrogen peroxide as the oxygen source, the reaction-controlled phase transfer catalyst catalyzes the epoxidation of chloropropene to prepare epichlorohydrin. The results of waste gas treatment in the reaction process are shown in Table 3. It can be seen that the organic matter in the waste gas after this process is greatly reduced, reducing the The content of allyl chloride in the exhaust gas.
[0039] Table 3. Waste gas treatment results of the epoxidation of propylene chloride to prepare epichlorohydrin
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com