Medical degradable high molecular antibacterial composite material
A composite material and polymer technology, which is applied in the field of degradable polymer medical materials, can solve the problems of resistance to tensile strength and other problems, achieve the effect of no allergic reaction, effective degradation, and guaranteed performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The preparation method of the biomass extract is as follows: soak mint dry powder in pepper water for 6-8 hours, then dry at a temperature of 60°C until the water content is lower than 5%, to obtain the extract A; then summer Wash the dried ears of subtilis subtilis, crush them through a 120-mesh sieve after drying, then mix them with ethanol solution with a mass concentration of 75-85% at a weight ratio of 1:4-6, and ultrasonicate for 60 minutes at a temperature of 35-45°C After suction filtration, ultrasonic treatment at 45°C for 1.5 hours, suction filtration, rotary evaporation and concentration at 50°C, and freeze-drying to obtain extract material B; extract material A and extract material B were mixed at a weight ratio of 2:1 to obtain raw material extract.
[0021] As can be seen from the above description, the extract A prepared by mint dry powder can effectively improve the antibacterial performance of the composite material, but it will affect the temperature r...
Embodiment 1
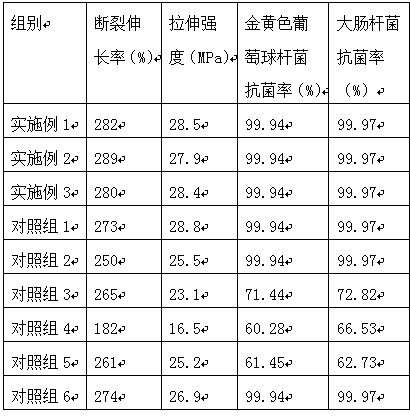
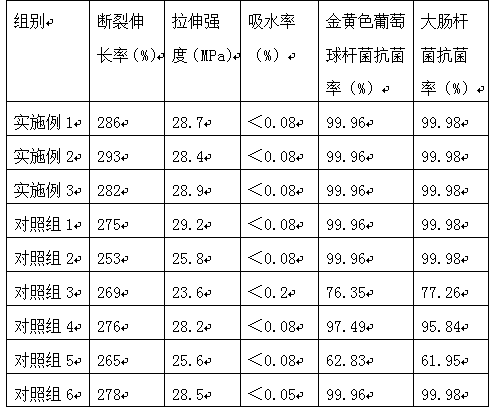
[0033]A medical degradable polymer antibacterial composite material, comprising the following raw materials in parts by weight: 55 parts of polytrimethylene glutarate with an intrinsic viscosity of 1.138dL / g, 2 parts of dimethylchlorosilane, 3-(2-pyridyl 5 parts of dithio)propionic acid N-hydroxysuccinimide ester, 11 parts of hydroxyl compound, 1 part of royal acid, 1.5 parts of toughening agent, 14 parts of biomass extract;
[0034] The preparation method of the biomass extraction material is as follows: soak the dry peppermint powder in pepper water for 7 hours, and then dry it at a temperature of 60°C until the water content is lower than 5%, to obtain the extraction material A; then the Prunella vulgaris Wash the dried fruit ears, crush them through a 120-mesh sieve after drying, and then mix them with an ethanol solution with a mass concentration of 80% at a weight ratio of 1:5. Under the condition of sonication for 1.5 hours, suction filtration, concentrated by rotary ev...
Embodiment 2
[0038] A medical degradable polymer antibacterial composite material, comprising the following raw materials in parts by weight: 50 parts of polytrimethylene glutarate with an intrinsic viscosity of 1.138dL / g, 3 parts of dimethylchlorosilane, 3-(2-pyridyl 7 parts of dithio)propionic acid N-hydroxysuccinimide ester, 7 parts of hydroxyl compound, 0.6 part of royal acid, 2 parts of toughening agent, 16 parts of biomass extract;
[0039] The preparation method of the biomass extract is as follows: soak the dry peppermint powder in pepper water for 8 hours, and then dry it at a temperature of 60°C until the water content is lower than 5%, so as to obtain the extract A; then the Prunella vulgaris Wash the dried fruit ears, crush them through a 120-mesh sieve after drying, and then mix them with an ethanol solution with a mass concentration of 85% at a weight ratio of 1:4. Under the condition of sonication for 1.5 hours, suction filtration, concentrated by rotary evaporation at 50°C,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| Functional group degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com