Crystal form, preparation and application of 4'-substituted nucleoside
A crystal form and crystal technology, applied in the field of medicinal chemistry, can solve the problems of no synthetic method and biological activity, unpredictable effect on HIV inhibitory activity, and reduced anti-HIV activity of tenofovir didronate diamide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the preparation of compound 1:
[0044] Compound 1 was prepared according to literature method (K. Fukuyama et al Org. Lett. 2015, 17, 828-831).
Embodiment 2
[0045] Embodiment 2, the preparation of compound Ia:
[0046]
[0047] Starting material 1 (9.64g, 15.97mmol, 1.0eq), pyridine (7.7mL, 95.8mmol, 6.0eq) was added to 100mL of dry toluene, and DAST (15.4g, 95.8mmol, 6.0eq) was dissolved in 40mL of dry In toluene, under nitrogen protection, add dropwise to the reaction solution at 0°C, after the dropwise addition is completed, rise to 50°C for reflux reaction for 6h, LC-MS detects that the reaction is complete, stop the reaction, cool down, add dropwise saturated sodium bicarbonate solution at low temperature to quench extinguished, adjusted the pH to 7-8, added EA for extraction, washed the organic phase with water, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, concentrated, PE beating, filtered to obtain 7.44g of crude compound 2, which was directly used in the following step. 1 H NMR (400MHz, CDCl 3 )δ8.01(d,J=2.0Hz,1H),7.44–7.28(m,10H),6.53(dd,J=11.4,5.1Hz,1H),5.86(s,2H),5...
Embodiment 3
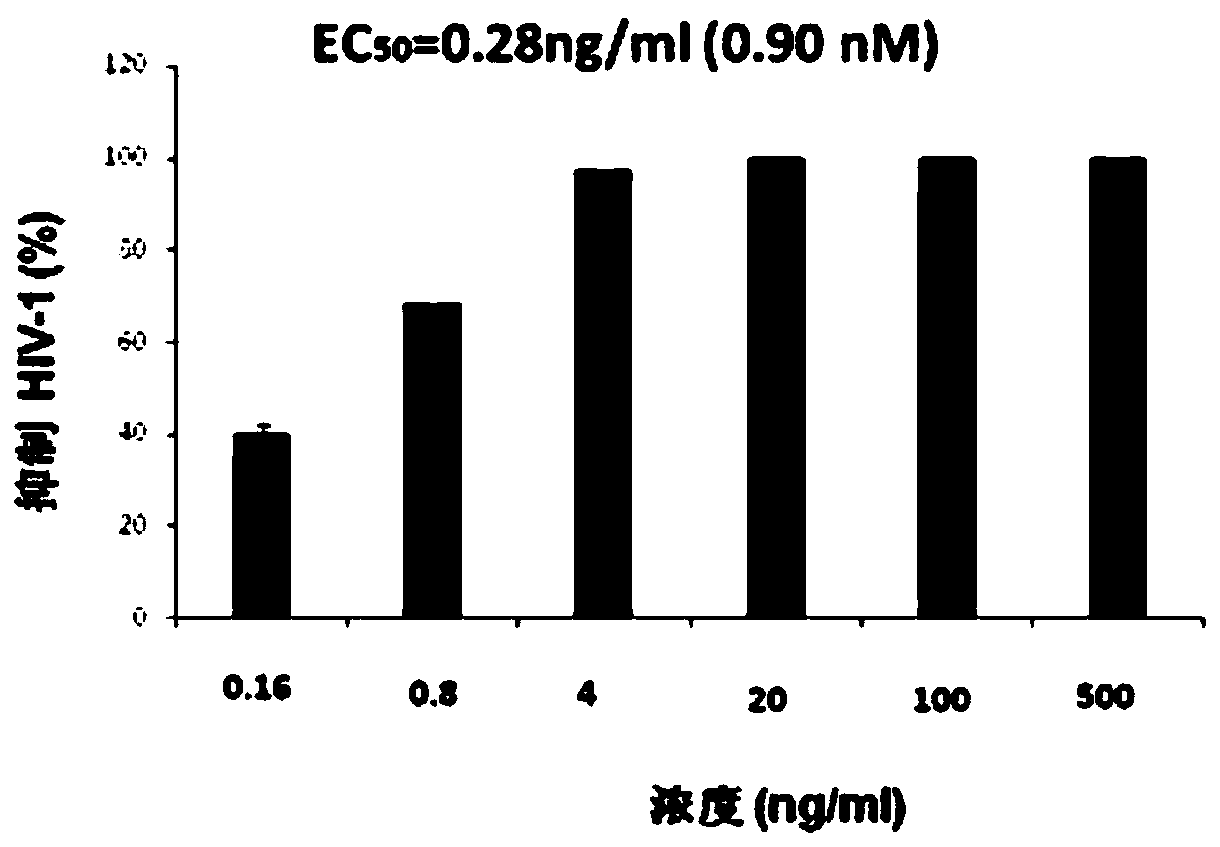
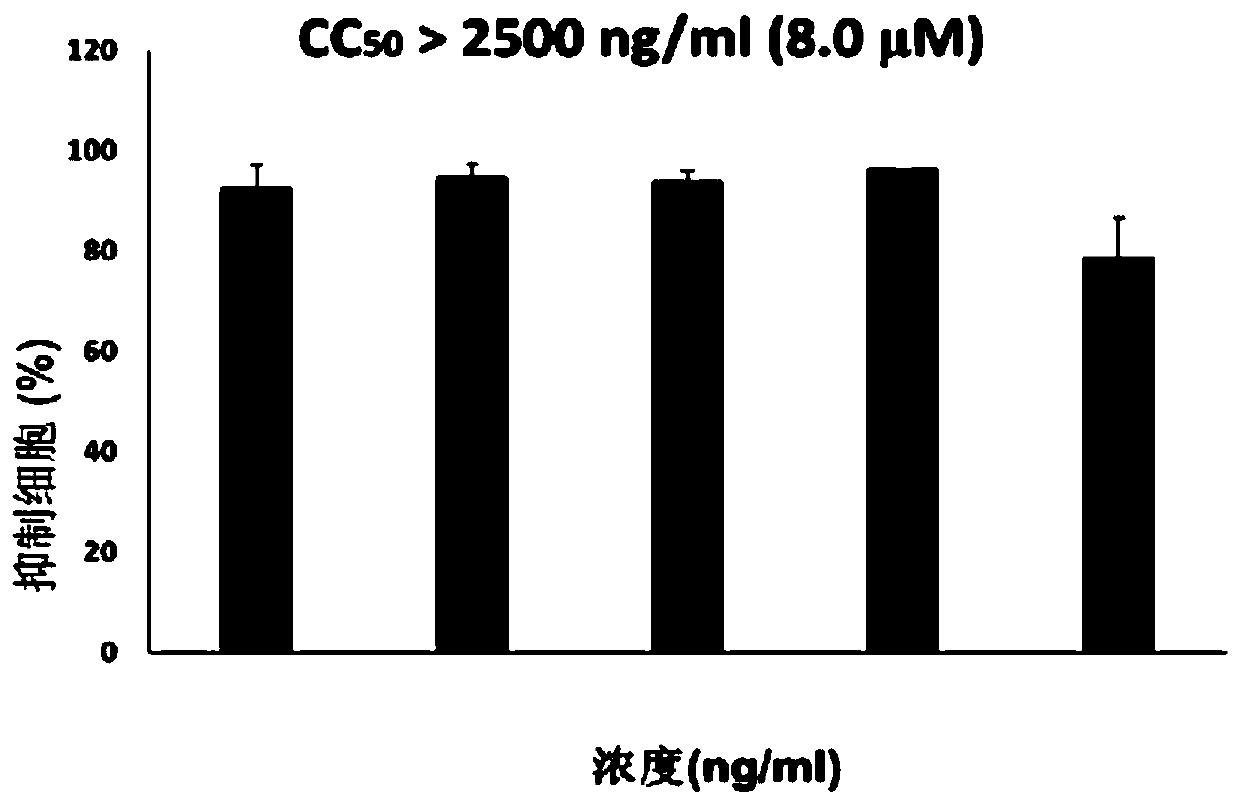
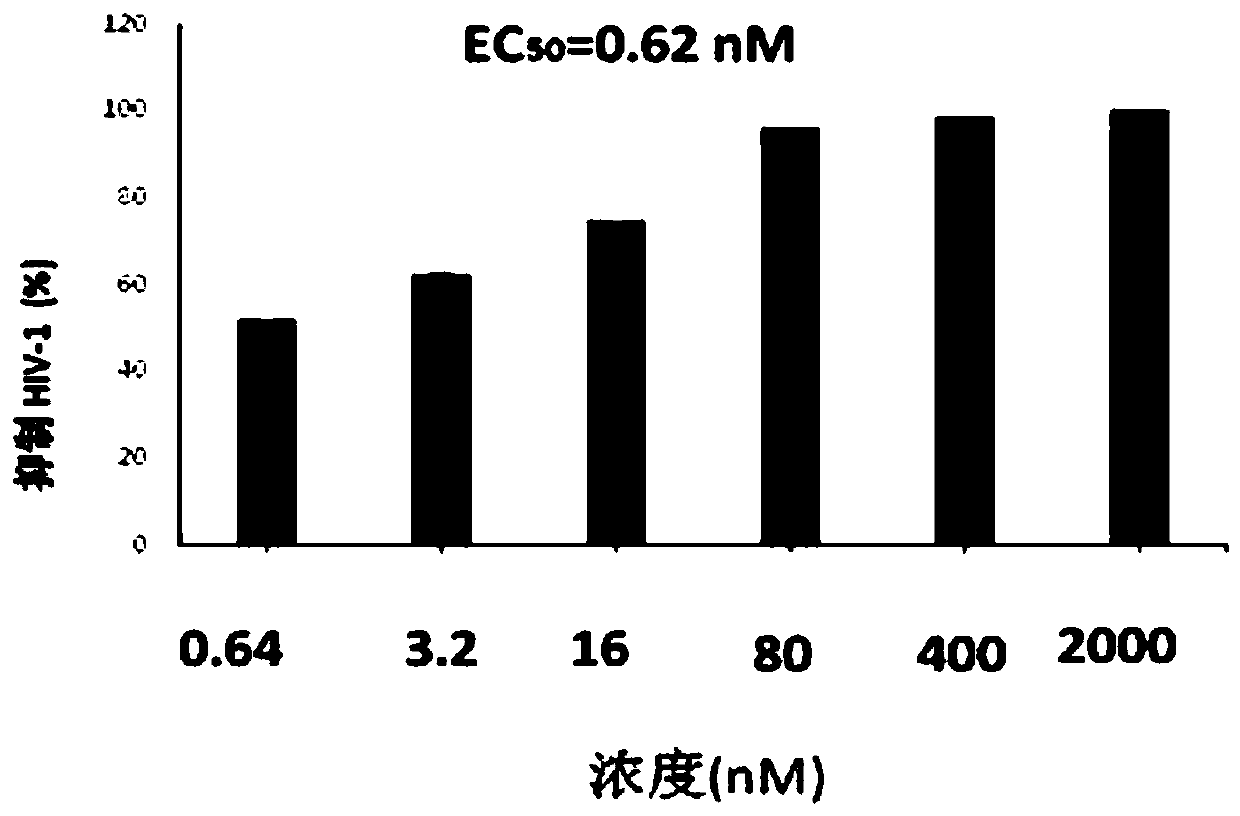
[0054] The assay material and method of embodiment 3, compound Ia and Ib antiviral activity
[0055] 3.1. Compounds:
[0056] Dissolve compound Ia or Ib in DMSO, store at -20°C, stock 10mg / ml;
[0057] 3.2. HIV-1 virus:
[0058] The plasmid used to package pseudotyped single-cycle infectious HIV-1 (HIV-luc / JRFL) is as follows: pLAI-Δenv-Luc contains the backbone gene sequence of HIV-1, but the env and vpr genes are deleted, and the nef gene locus is inserted Luciferase reporter gene; pJRFL contains the env gene of CCR5 tropism HIV-1; plasmid co-transfection into HEK293T cells. Cell supernatants were collected and aliquoted by filtration. p24 gag capture ELISA for quantification.
[0059] 3.3. HIV-1 infection detection:
[0060] CD4 + T cells (Hut / CCR5) were cultured in complete 1640 medium (Gibco) containing 10% fetal bovine serum (Gibco), 100 U / ml penicillin (Invitrogen) and 100 U / ml streptomycin (Invitrogen) . 1×10 5 Hut / CCR5 cells were added with test compound Ia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com