Synthesis method of 1,3-butadiene and preparation method of catalyst
A synthesis method and catalyst technology, applied in the field of 1,3-butadiene synthesis, can solve the problems of low specific surface area of oxides, difficulty in industrial scale-up, and lack of shape selectivity, etc., and achieve simple preparation process, easy industrialization, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
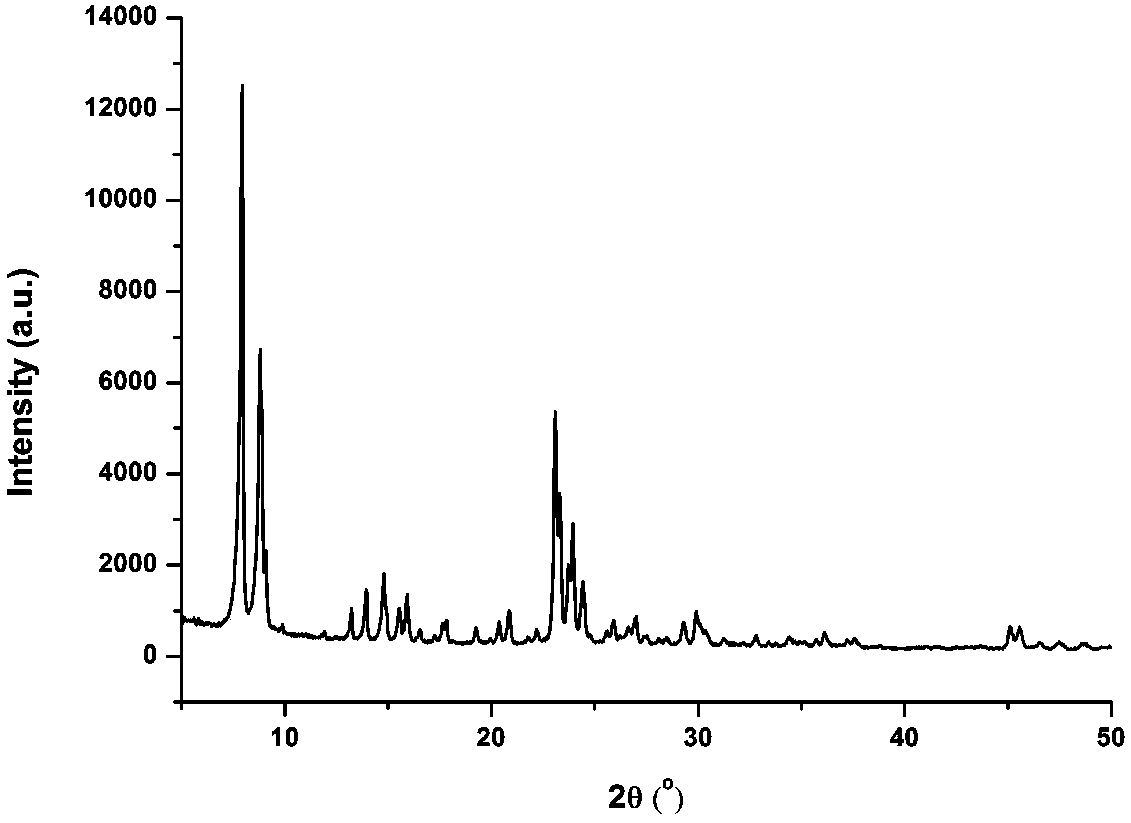
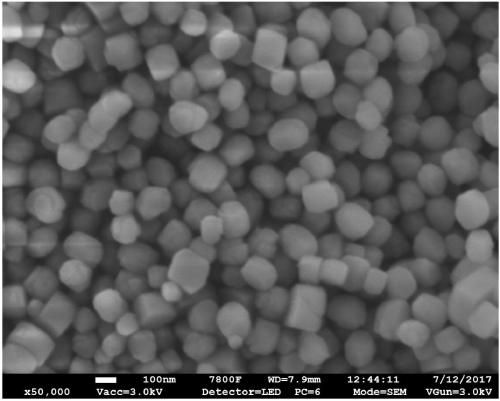
[0024] 1. Preparation of catalyst
[0025] (1) According to the consumption of 3wt%Zn-10wt%Y-MFI, Zn(NO 3 ) 2 6H 2 O and Y (NO 3 ) 3 6H 2 O was dissolved in 30mL of water, the concentration of zinc nitrate in the solution was 0.3wt%, under stirring conditions, ethyl orthosilicate was added dropwise in the above solution, the molar concentration of silicon in the solution was 1.6mol / L, and stirred for 3h, Then add 25wt% tetrapropylammonium hydroxide aqueous solution dropwise, the addition amount is 1.6mol, stir 12h;
[0026] (2) Put the obtained gel into a stainless steel hydrothermal kettle and crystallize at 160°C for 3 days;
[0027] (3) The obtained white powder was centrifuged, dried at 150°C for 12h, and calcined at 600°C for 5h to obtain a 3wt%Zn-10wt%Y-MFI catalyst, abbreviated as 3Zn-10Y-MFI.
[0028] 2. Molecular sieve catalyst catalyzes the conversion of ethanol to butadiene
[0029] The resulting 1g 3Zn-10Y-MFI catalyst was loaded into a φ6 stainless steel...
Embodiment 2
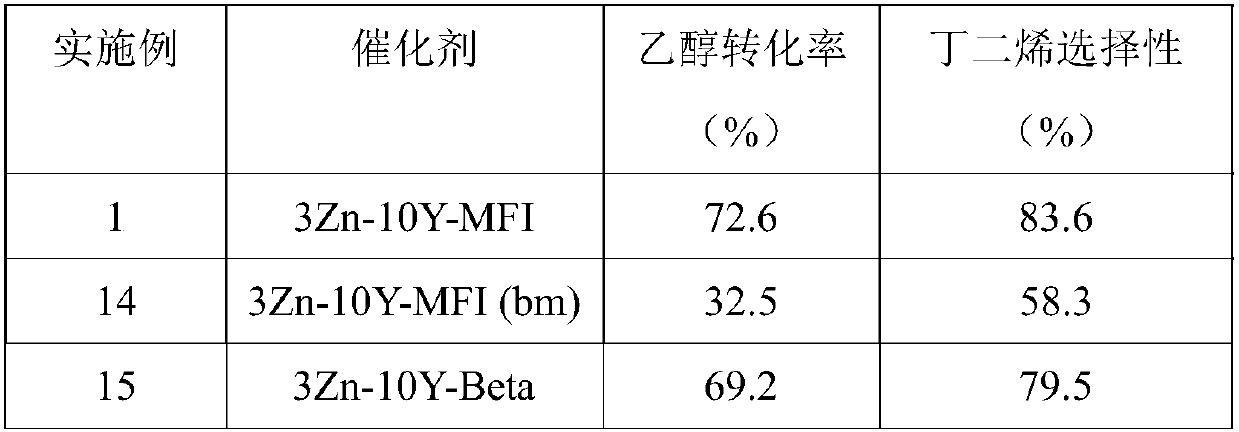
[0035] The difference between Example 2 and Example 1 is that the prepared catalyst is a 3wt%Zn-10wt%Zr-MFI catalyst, abbreviated as 3Zn-10Zr-MFI, and other reaction conditions are the same as in Example 1. The specific experimental results are shown in Table 1.
Embodiment 3
[0037] The difference between Example 3 and Example 1 is that the prepared catalyst is a 3wt%Zn-10wt%Hf-MFI catalyst, abbreviated as 3Zn-10Hf-MFI, and other reaction conditions are the same as in Example 1. The specific experimental results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com