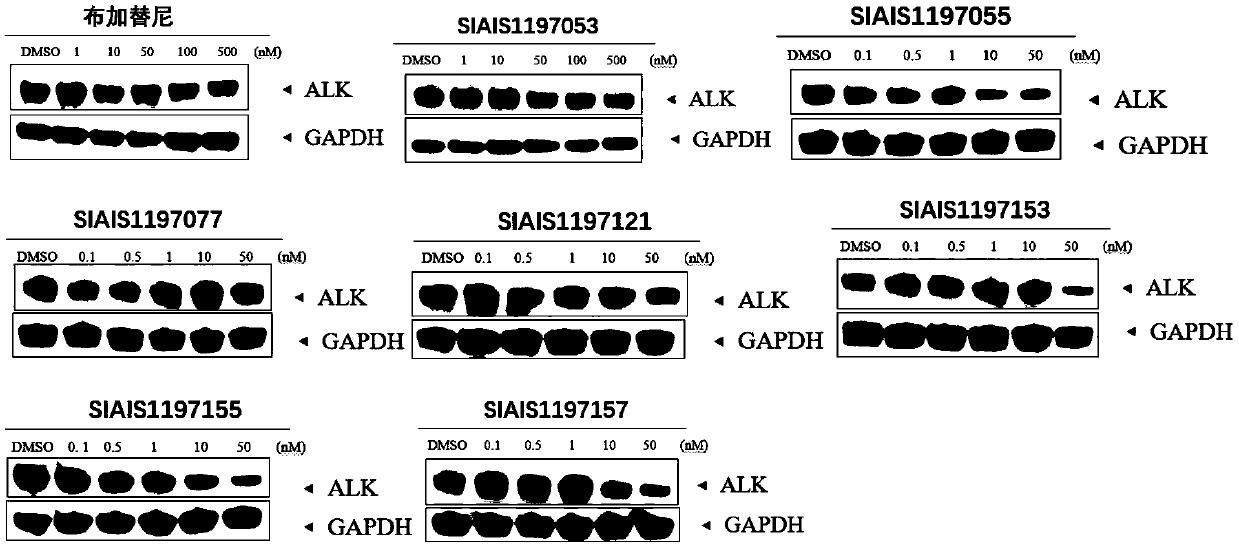
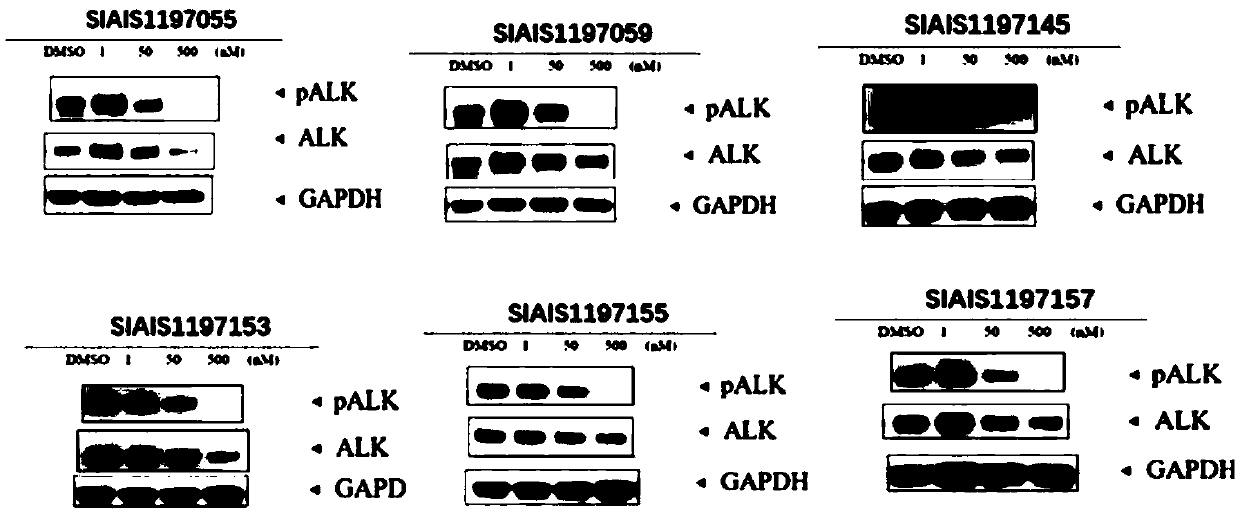
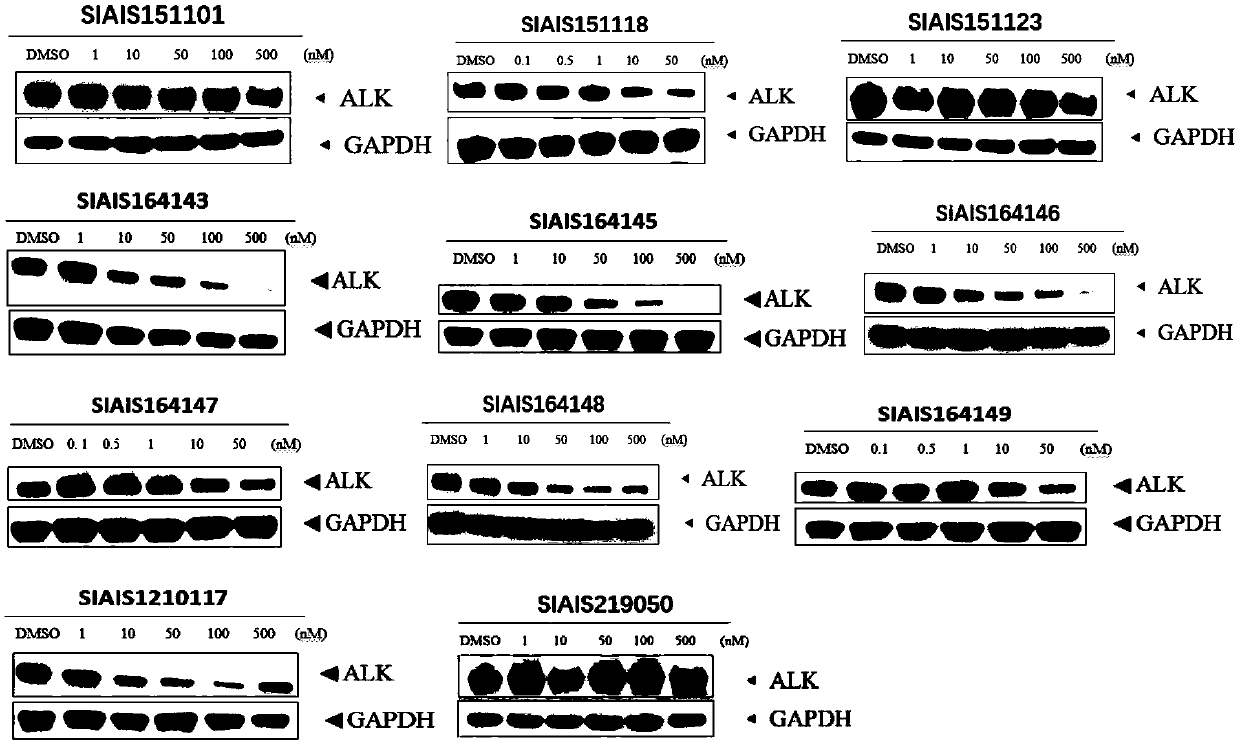
ALK (anaplastic lymphoma kinase) protein degradation agent and anti-tumor application thereof
An inhibitor and solvate technology, applied in the field of ALK protein degrader and its anti-tumor application, can solve the problems of ALK protein drug resistance and inability to clear ALK protein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0277] In the following description, numerous specific details are set forth in order to provide a thorough understanding of the invention. The present invention may be practiced without some or all of these specific details. In other instances, well known process operations have not been described in detail in order not to unnecessarily obscure the present invention. While the invention will be described in conjunction with specific embodiments, it will be understood that they are not intended to limit the invention to those embodiments.
[0278] The following abbreviations are used throughout the specification and examples:
[0279] Boc tert-butoxycarbonyl
[0280] Con. Concentration
[0281] DCM dichloromethane
[0282] DMF N,N-Dimethylformamide
[0283] DMSO dimethyl sulfoxide
[0284] DIPEA N,N-Diisopropylethylamine
[0285] EDCI carbodiimide
[0286] ESI electrospray ionization
[0287] equiv equivalent
[0288] EtOH ethanol
[0289] HOAT 1-Hydroxy-7-azobenzot...
preparation Embodiment 1
[0357] Intermediate Preparation Example 1: Preparation of Brigatinib Derivative A (SIAIS1197135)
[0358] Brigatinib derivative A (SIAIS1197135) was prepared according to Scheme 1.
[0359] Preparation of tert-butyl 4-(3-methoxy-4-nitrophenyl)piperazine-1-carboxylate (SIAIS1197111):
[0360] Under open conditions, 5-fluoro-2-nitroanisole (7g, 40.9mmol) was dissolved in 60mL of DMF solution, followed by adding K 2 CO 3 (8.4g, 60.8mmol), N-tert-butoxycarbonylpiperazine (9.1g, 48.9mmol), stirred overnight at room temperature. After the reaction was completed, quenched with water, extracted with ethyl acetate, washed the organic phase with water, washed with saturated brine, dried over anhydrous sodium sulfate, spin-dried, beating with a mixed solvent of petroleum ether:ethyl acetate=5:1, and filtered through a sand core 11.1 g of yellow target solid SIAIS1197111 was obtained with a yield of 80%. 1 H NMR (500MHz, DMSO) δ7.89(d, J=9.3Hz, 1H), 6.57(d, J=9.5 Hz, 1H), 6.52(s, 1H),...
preparation Embodiment 2
[0366] Intermediate Preparation Example 2: Preparation of Brigatinib Derivative B
[0367] Using a method similar to that of the brigatinib derivative A in Example 1 of intermediate preparation, the brigatinib derivative B was prepared according to Scheme 1, and the intermediate synthesis data and structural characterization data are as follows:
[0368]
[0369] tert-Butyl(1-(3-methoxy-4-nitrophenyl)piperidin-4-yl)carbamate (SIAIS151054).(Yellow solid, 1.81g, 88%) 1 H NMR (500MHz, CDCl 3 )δ7.99(t, J=8.9Hz, 1H), 6.41(dd, J=9.4, 2.5Hz, 1H), 6.30(d, J=2.5Hz, 1H), 4.49(s, 1H), 3.94( s,3H),3.86–3.82(m,2H),3.71(s,1H),3.09–3.00(m,2H),2.11–2.03(m,2H),1.89–1.75(m,2H),1.45( s,9H).
[0370]
[0371] tert-Butyl(1-(4-amino-3-methoxyphenyl)piperidin-4-yl)carbamate (SIAIS151062).(gray purple solid, 411.6mg, 90%) 1 H NMR (500MHz, DMSO) δ6.82(d, J=7.6Hz, 1H), 6.50(d, J=8.5Hz, 1H), 6.48(d, J=2.5Hz, 1H), 6.28(dd, J =8.4,2.4Hz,1H),4.20(s,2H),3.73(s,3H),3.33–3.26(m,3H),2.56–2.50(m,2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com