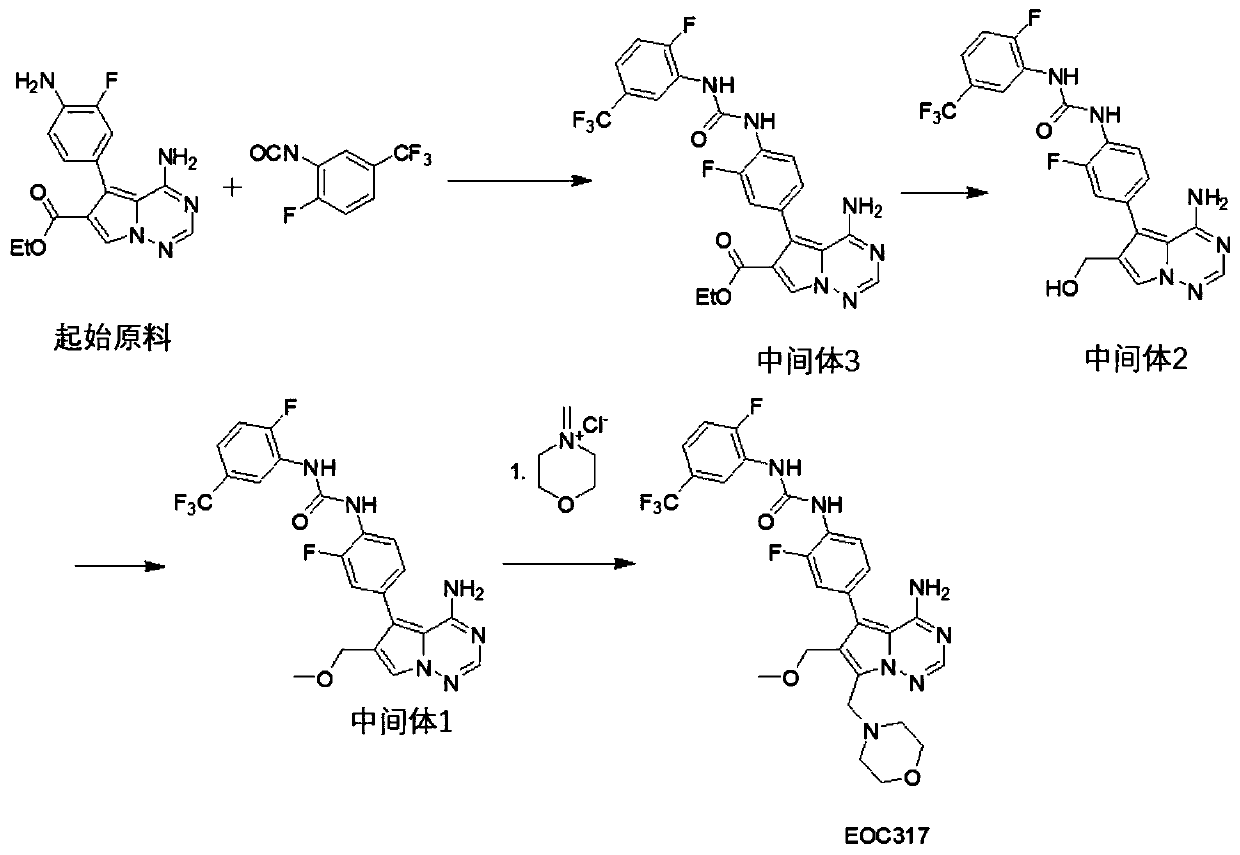
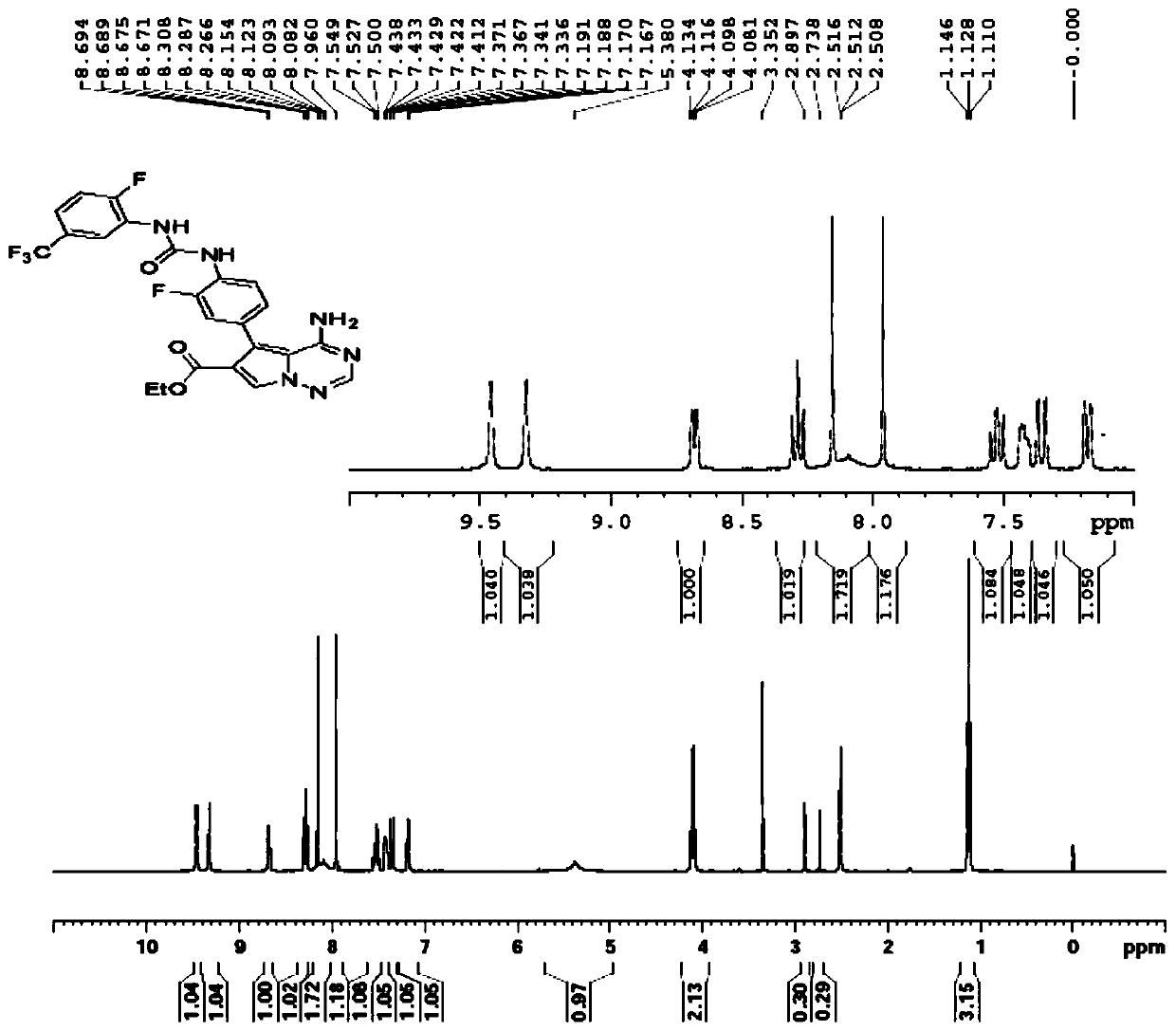
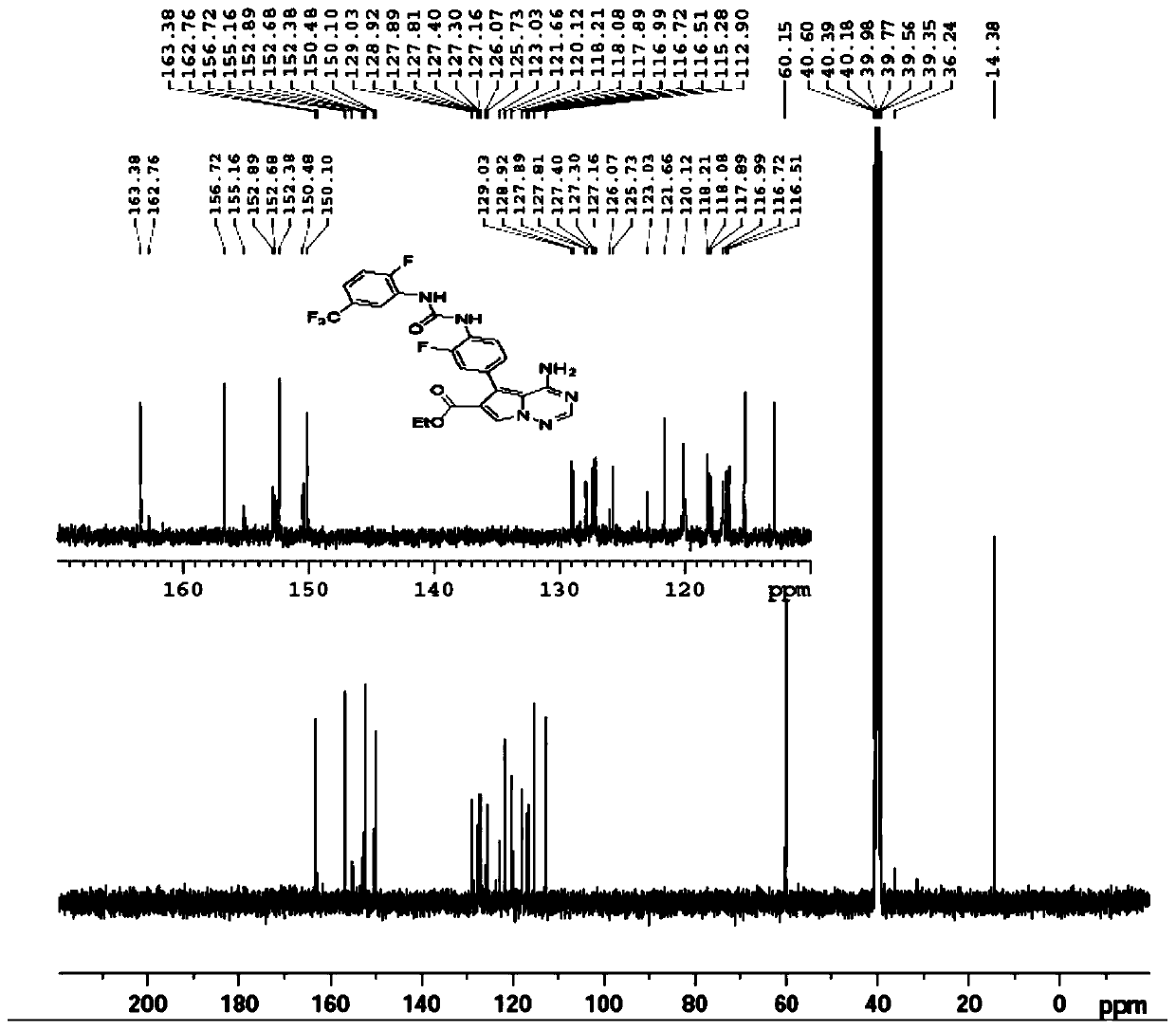
Method for industrially producing EOC 317
A technology of methyl etherification and intermediates, applied in the field of pharmaceutical production, can solve the problems of long preparation time, high preparation cost, unsuitable production, etc., and achieve the effects of low production cost, short preparation cycle and safe production method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1E
[0130] The synthesis of embodiment 1EOC317
[0131] 1.1 Synthesis of Intermediate 3
[0132] Add 2.0L dichloromethane to a 5.0L glass reactor, add 4-amino-5-(4-amino-3-fluorophenyl)pyrrole[1,2-f][1,2,4]triazine- 6-Carboxylic acid ethyl ester (250.0g, 0.79mol), the reaction system was cooled to 5-10°C under the protection of nitrogen, and 2-fluoro-5-(trifluoromethyl)benzene isocyanate (324.1g, 1.58mol) was added dropwise ), the temperature was controlled below 15°C during the dropping process, and the temperature of the reaction system was raised to 25-30°C after the dropwise addition. During this process, the reaction system gradually turned into a brown solution. Gradually viscous, monitored by HPLC to ethyl 4-amino-5-(4-amino-3-fluorophenyl)pyrrole[1,2-f][1,2,4]triazine-6-carboxylate After the reaction is complete, add 2.0L of dimethyl sulfoxide to the reaction system, add 350mL of 2.1N hydrochloric acid, raise the temperature of the reaction system to 75-80°C, steam until...
Embodiment 2E
[0152] The synthesis of embodiment 2EOC317
[0153] 2.1 Synthesis of intermediate 3
[0154] Add 2.0L tetrahydrofuran to a 5.0L glass reactor, add 4-amino-5-(4-amino-3-fluorophenyl)pyrrole[1,2-f][1,2,4]triazine-6-carboxy Ethyl acetate (250.0g, 0.79mol), the reaction system was cooled to 5-10°C under the protection of nitrogen, and 2-fluoro-5-(trifluoromethyl)benzene isocyanate (405.1g, 1.98mol) was added dropwise. During the addition process, the temperature should be controlled below 15°C. After the dripping, the temperature of the reaction system was raised to 25-30°C. During this process, the reaction system gradually turned into a brown solution. After stirring for about 20 minutes, white solids gradually precipitated in the system, and gradually became Viscous, HPLC monitoring to 4-amino-5-(4-amino-3-fluorophenyl) pyrrole [1,2-f] [1,2,4] triazine-6-carboxylic acid ethyl ester reaction is complete, Add 2.0L of N,N-dimethylformamide to the reaction system, add 350mL of 2....
Embodiment 3E
[0170] The synthesis of embodiment 3EOC317
[0171] 3.1 Synthesis of Intermediate 3
[0172] Add 2.0L tetrahydrofuran to a 5.0L glass reactor, add 4-amino-5-(4-amino-3-fluorophenyl)pyrrole[1,2-f][1,2,4]triazine-6- Ethyl carboxylate (250.0g, 0.79mol), the reaction system was cooled to 5-10°C under nitrogen protection, and 2-fluoro-5-(trifluoromethyl)phenylisocyanate (453.7g, 2.21mol) was added dropwise, During the dropping process, the temperature was controlled below 15°C. After the dropping, the temperature of the reaction system was raised to 25-30°C. During this process, the reaction system gradually turned into a brown solution. After stirring for about 20 minutes, a white solid gradually precipitated in the system and gradually turned into Viscous, HPLC monitoring to 4-amino-5-(4-amino-3-fluorophenyl)pyrrole[1,2-f][1,2,4]triazine-6-carboxylate ethyl ester reaction completed , add 2.0L of dimethyl sulfoxide to the reaction system, add 350mL of 2.1N hydrochloric acid, rai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com