Dimethrin microcapsules, preparation method therefor and insectproof preparation
A technology of microcapsules and pyrethroids, applied in the fields of botanical equipment and methods, pesticides, biocides, etc., can solve the problems of damage to human health and ecological protection, unsafe storage, transportation and use, and poor storage stability of preparations. Achieving good controlled release effect, reducing medication frequency and dosage, and good storage stability at room temperature and high temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
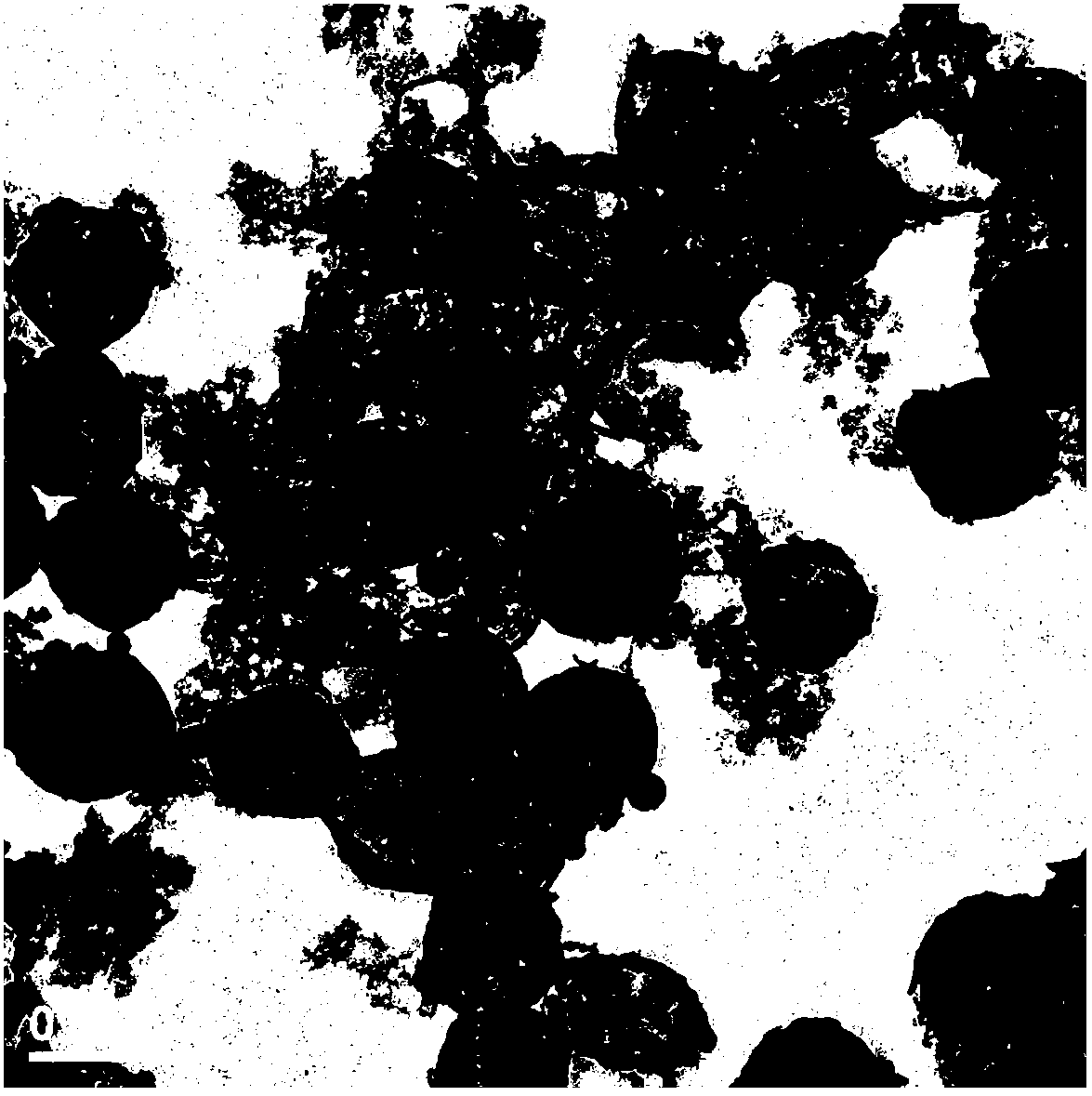
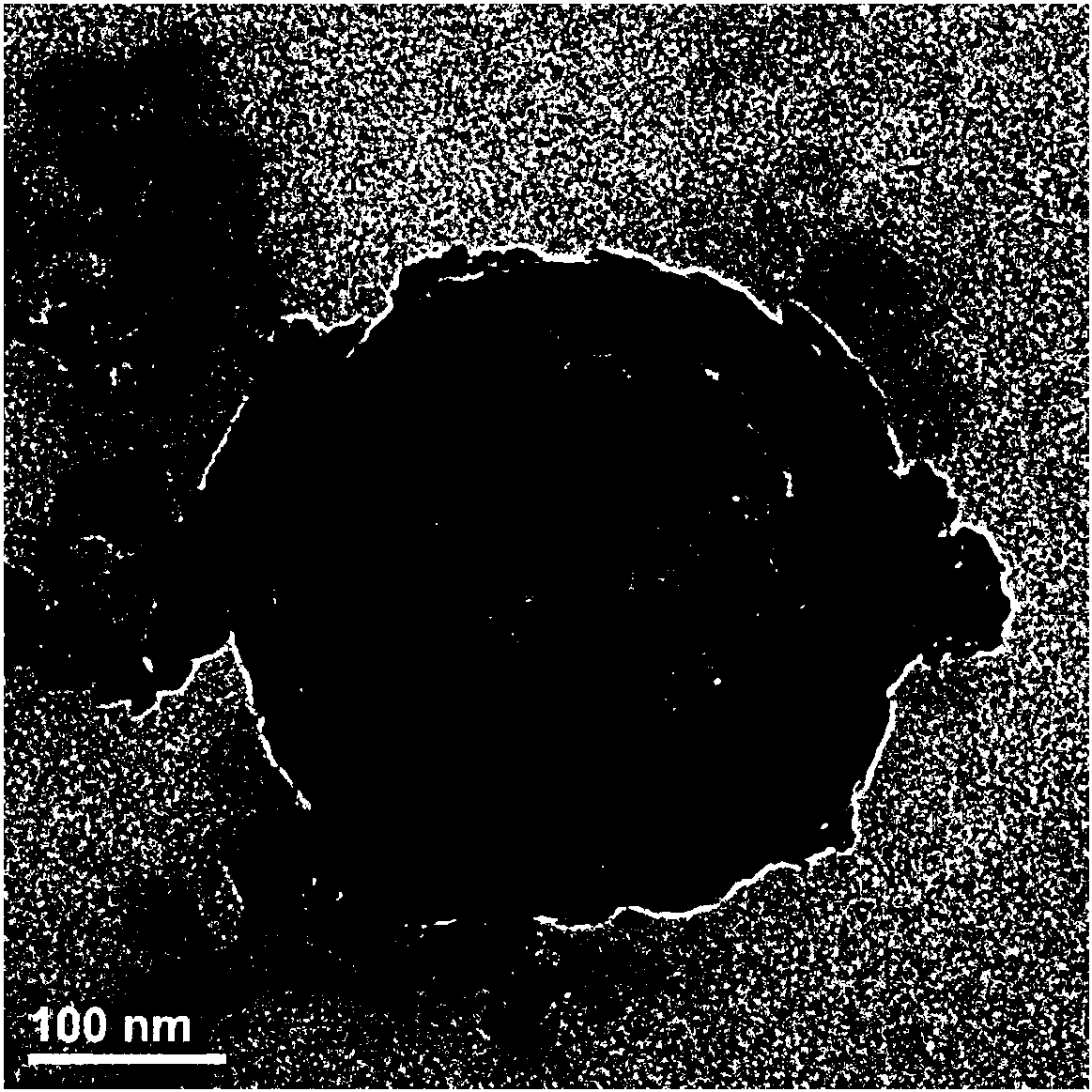
[0050] (1) Prepare 10mmol / L Tris buffer solution and adjust the pH to about 8.5 with 0.5mol / L HCl solution. 60mgSiO 2 The particles were pretreated with 10mmol / LTris buffer solution, centrifuged and redispersed. The treated particles were added to 15mL of 10mmol / L Tris buffer solution, and then 30mg of dopamine was added and stirred for 24 hours. The product was centrifuged, washed with Tris buffer solution and redistilled water, and centrifuged until the supernatant was colorless.
[0051] (2) Add the product obtained in (1) to 2mol / L hydrofluoric acid / 8mol / L ammonium fluoride buffer solution, magnetically stir for 12 hours, remove the template material, and rinse with redistilled water, vacuum at 40°C Dry for 12 hours.
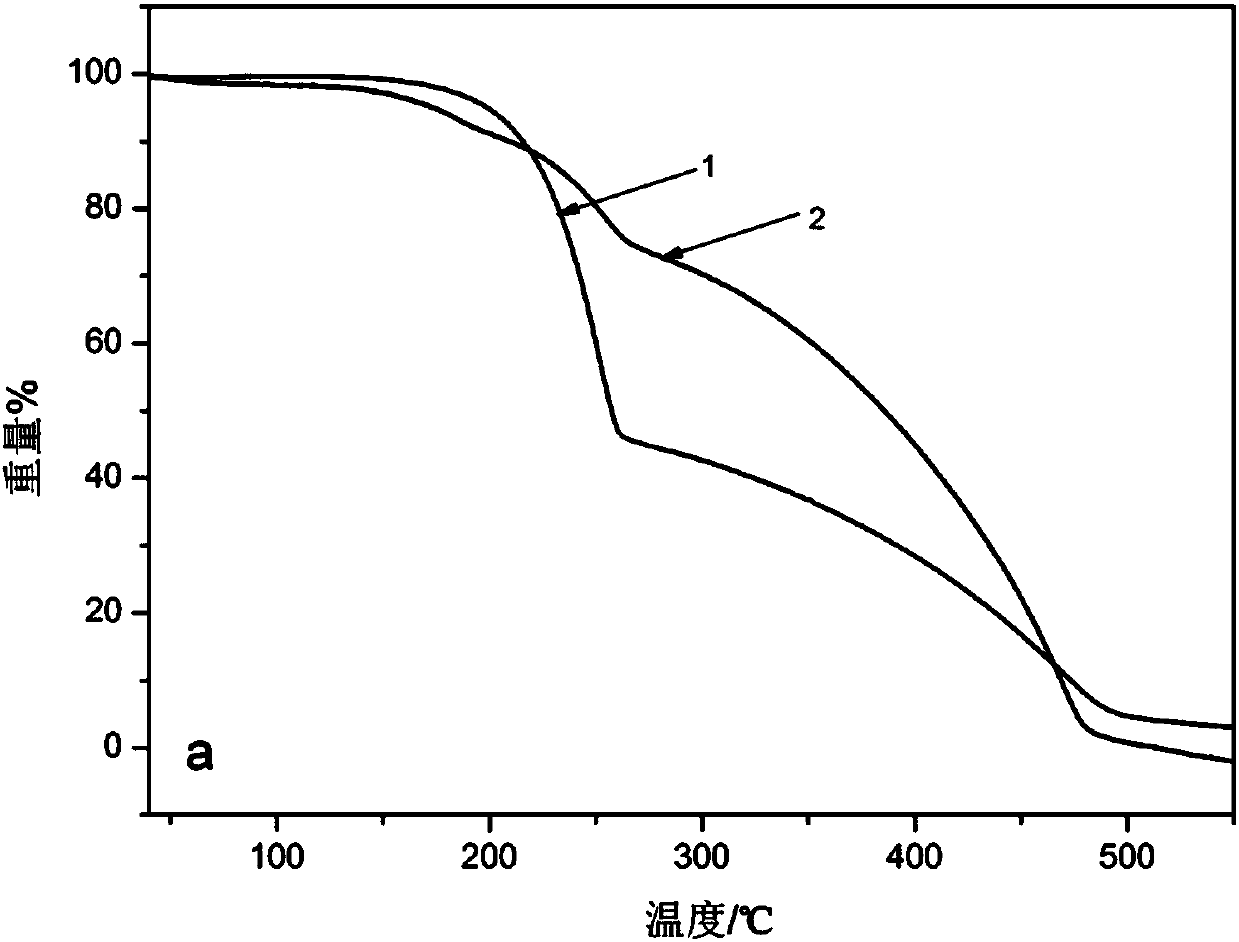
[0052] (3) Add 30 mg of the product obtained in (2) to 45 mL of 10 mg / mL lambda-cyhalothrin dissolved in N,N-dimethylformamide to form a pyrethroid solution, magnetically stir for 24 hours, and centrifuge. And repeatedly washed with double distilled water, the...
Embodiment 2
[0055] (1) Prepare 10mmol / L Tris buffer solution and adjust the pH to about 8.5 with 0.5mol / L HCl solution. 60mgSiO 2 The particles were pretreated with 10mmol / LTris buffer solution, centrifuged and redispersed. The treated particles were added to 15mL of 10mmol / L Tris buffer solution, and then 30mg of dopamine was added and stirred for 24 hours. The product was centrifuged, washed with Tris buffer solution and redistilled water, and centrifuged until the supernatant was colorless.
[0056] (2) Add the product obtained in (1) to 2mol / L hydrofluoric acid / 8mol / L ammonium fluoride buffer solution, magnetically stir for 12 hours, remove the template material, and rinse with redistilled water, vacuum at 40°C Dry for 12 hours.
[0057] (3) Add 30 mg of the product obtained in (2) to 45 mL of 20 mg / mL lambda-cyhalothrin dissolved in N,N-dimethylformamide to form a pyrethroid solution, magnetically stir for 24 hours, and centrifuge. And repeatedly washed with double distilled water, the...
Embodiment 3
[0060] (1) Prepare 10mmol / L Tris buffer solution and adjust the pH to about 8.5 with 0.5mol / L HCl solution. 60mgSiO 2 The particles were pretreated with 10mmol / L Tris buffer solution, centrifuged and redispersed. The treated particles were added to 15mL of 10mmol / L Tris buffer solution, and then 30mg of dopamine was added and stirred for 24 hours. The product was centrifuged, washed with Tris buffer solution and redistilled water, and centrifuged until the supernatant was colorless.
[0061] (2) Add the product obtained in (1) to 2mol / L hydrofluoric acid / 8mol / L ammonium fluoride buffer solution, magnetically stir for 12 hours, remove the template material, and rinse with redistilled water, vacuum at 40°C Dry for 12 hours.
[0062] (3) Add 30 mg of the product obtained in (2) to 45 mL of 30 mg / mL lambda-cyhalothrin dissolved in N,N-dimethylformamide to form a pyrethroid solution, magnetically stir for 24 hours, and centrifuge. And repeatedly washed with double distilled water, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com