Amlodipine besylate tablets
A technology of amlodipine besylate tablets and amlodipine besylate, which is applied in the field of medicine, can solve the problem of affecting product yield, active ingredient content and dissolution rate, level to light, humidity, heat instability, and affecting products. Dissolution and other issues, to achieve the effect of improving content uniformity and dissolution, ensuring dissolution and high dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
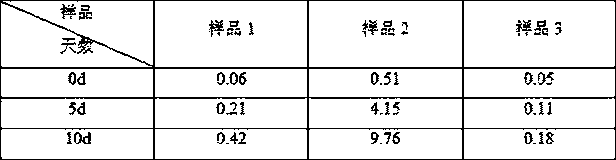
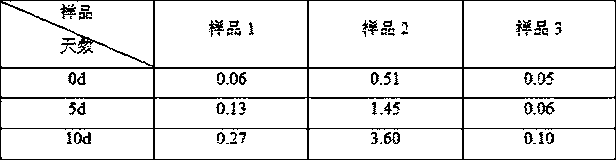
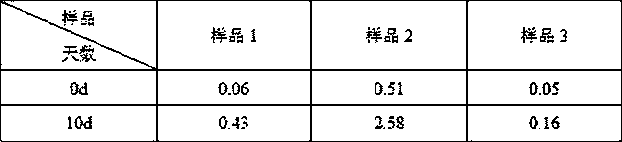
Image
Examples
Embodiment 1
[0041] Amlodipine besylate sheet, is characterized in that, described amlodipine besylate sheet comprises the component of following percentage by weight:
[0042] Amlodipine besylate 2%-5%
[0043] Micropowder silica gel 0.1%-1%
[0044] Microcrystalline cellulose 55%-70%
[0045] Anhydrous calcium hydrogen phosphate 25%-35%,
[0046] Magnesium stearate 2%-4%,
[0047] Sodium carboxymethyl starch 0.1%-1%.
[0048] Amlodipine Besylate Tablet of the present invention comprises the following components by weight percentage:
[0049] Amlodipine Besylate 2.6%,
[0050] Micropowder silica gel 0.6%,
[0051] Microcrystalline Cellulose 65%,
[0052] Dicalcium Phosphate Anhydrous 30%,
[0054] Sodium carboxymethyl starch 0.6%
[0055] Amlodipine Besylate Tablet of the present invention comprises the following components by weight percentage:
[0056] Amlodipine Besylate 3.5%,
[0057] Micropowder silica gel 0.5%,
[0058] Microcrystalline C...
Embodiment 2
[0092] Amlodipine besylate sheet, the amlodipine besylate sheet comprises the following components by weight percentage:
[0093] Amlodipine Besylate 2.5%,
[0094] Micropowder silica gel 0.5%,
[0095] Microcrystalline Cellulose 62%,
[0096] Dicalcium Phosphate Anhydrous 32%,
[0097] Magnesium stearate 2% added,
[0098] Sodium carboxymethyl starch 0.5%,
[0099] Plus magnesium stearate 0.5%.
[0100] The preparation method of the amlodipine besylate sheet of the present embodiment may further comprise the steps:
[0101] (1) Take the raw and auxiliary materials in the above formula, first mix amlodipine besylate and micropowder silica gel evenly to obtain the mixed material A, and then pass through a 120-mesh sieve; then pass anhydrous calcium hydrogen phosphate through a 200-mesh sieve, microcrystalline Cellulose is passed through a 100-mesh sieve, sodium carboxymethyl starch and magnesium stearate are passed through a 80-mesh sieve;
[0102] (2) Dissolving microcr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com