Pharmaceutical application of 2-(4-piperidinylstyrene)-1,3,3-trimethyl-3h-indolium iodide
A technology based on styrene and trimethyl, applied in the field of fluorescent compound-2--1,3,3-trimethyl-3H-indolium iodide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
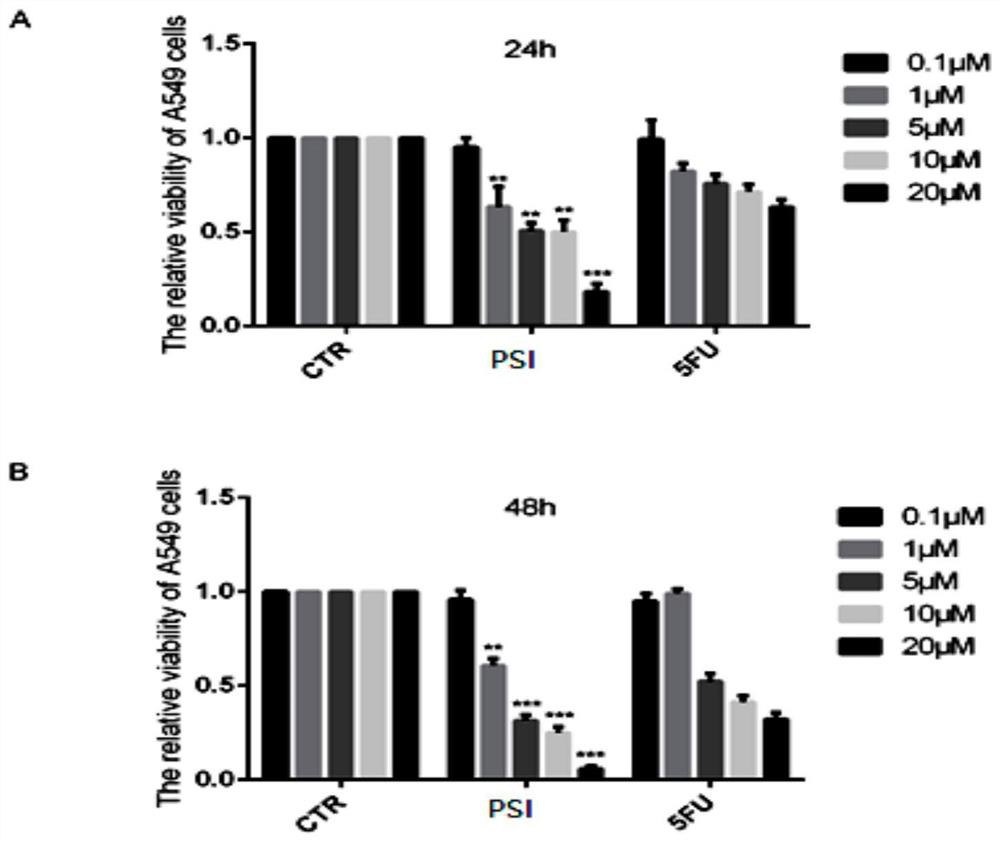
[0053] Example 1: Detection of survival rate by SRB method after PSI treatment of A549 cells.
[0054] (1) Compound PSI was added to treat the cells pre-planted in 96-well plate. (2) After compound treatment for 24 hours and 48 hours, the old culture medium was discarded, 100 μL of trichloroacetic acid was added, and the cells were fixed at 4° C. for 1 hour. (3) Discard the fixative. Wash five times with double-distilled water in gentle motions, and dry at room temperature. (4) Add 50 μL of SRB to each well, shake at room temperature for 10 minutes. (5) Wash 5 times with 1% acetic acid and dry at room temperature. (6) Add 100 μL Tris base in non-buffer solution with a concentration of 10 mM / L to each well and place on a shaker for 10 minutes. (7) Select 540nm excitation light to measure OD value.
[0055] The results show that: PSI can reduce the survival rate of A549 cells in a concentration-dependent manner after 24h and 48h of cells, see figure 1 .
Embodiment 2
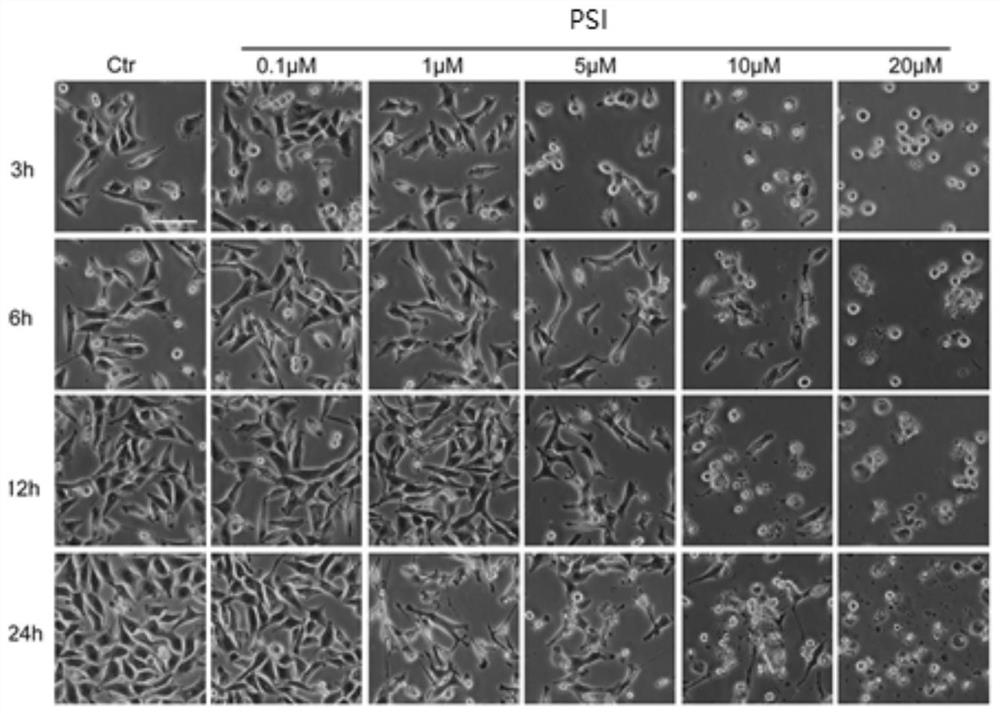
[0056] Example 2: After PSI treatment of A549 cells, detection of morphological changes and apoptosis levels.
[0057] A549 cells were seeded in a 6 cm-diameter petri dish at 37°C in CO 2 After being cultured in the incubator for 12 hours, PSI was added for 3 hours, 6 hours, 12 hours and 24 hours, and the cell morphology was photographed under an inverted microscope.
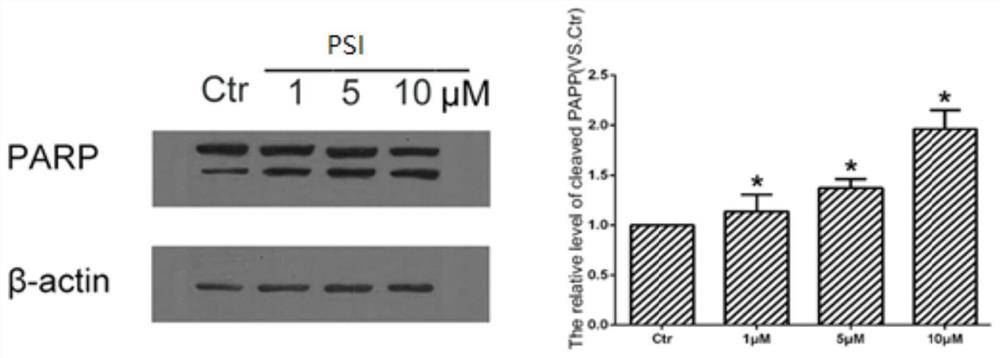
[0058] A549 cells were seeded in a 6 cm-diameter petri dish at 37°C in CO 2 After culturing in the incubator for 12 hours, add PSI to treat for 24 hours, lyse the cells, collect the cell lysate, centrifuge at 12000g for 15 minutes at 4°C, take the supernatant, and use western blot to detect the level of PARP protein cleavage in different cells.
[0059] The results showed that after treating A549 cells with different concentrations of PSI for different times, the density of cells could be significantly reduced and the release of apoptotic bodies could be promoted; after treatment of A549 cells with different co...
Embodiment 3
[0060] Example 3: Western blot detection of autophagy level after PSI treatment of A549 cells.
[0061] A549 cells were seeded in a 6 cm-diameter petri dish at 37°C in CO 2 After culturing in the incubator for 12 hours, add PSI to treat for 24 hours, lyse the cells, collect the cell lysate, centrifuge at 12,000g for 15 minutes at 4°C, take the supernatant, and use western blot to detect LC3-Ⅰ, LC3-Ⅱ and P62 proteins in differently treated cells Level.
[0062] The results show that: A549 cells treated with different concentrations of PSI can significantly increase the protein level of LC3-II and reduce the protein level of P62, see Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com