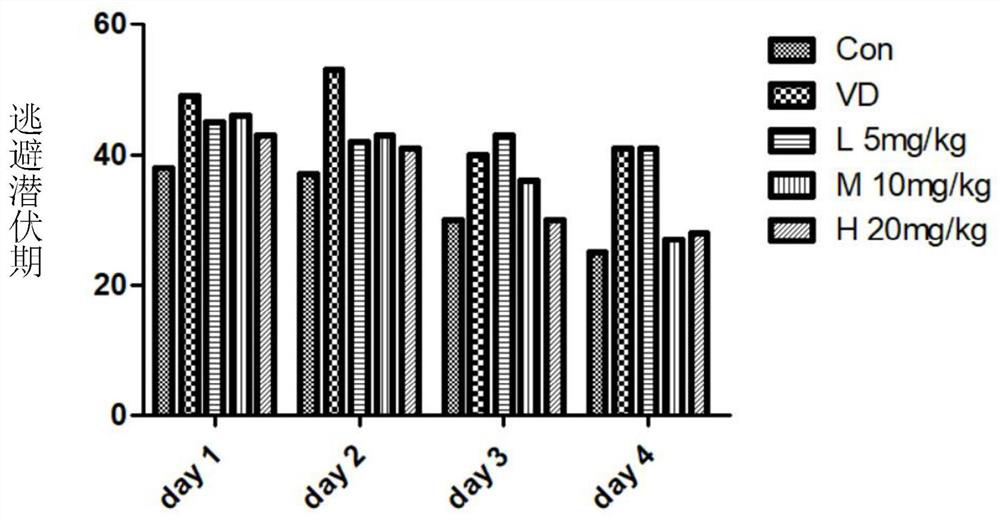
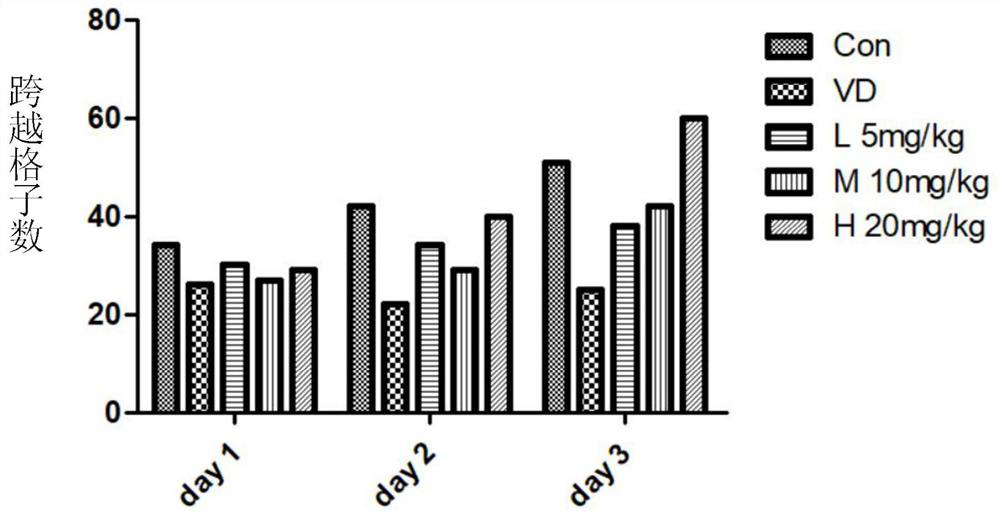
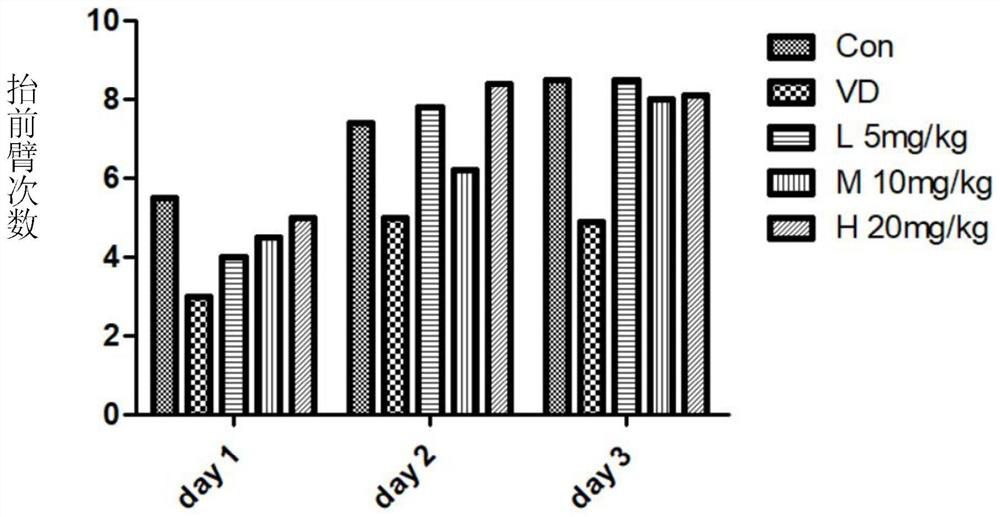
α-Neebisin derivatives and their preparation methods and applications
A technology of azadirachtin and derivatives, applied in the field of medicine, can solve the problems of difficult to achieve therapeutic effect, no effect of treating cerebral infarction or vascular dementia, etc., and achieve high safety, good therapeutic effect, and the effect of reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis (synthetic route figure such as figure 1 shown)
[0039]
[0040] Take α-Neebisin (40mg, 0.1mmol) in toluene solution, stir until dissolved, the solution turns yellow at this time, add 2,3-dichloro-5,6 dicyanoquinone (DDQ, 45mg, 0.2mmol) , reacted at 40°C, detected by TLC during the reaction, the reaction was completed after 5 hours, the toluene was distilled off under reduced pressure, separated and purified by silica gel column chromatography, and the yellow solid compound HZA was obtained with a yield of 85%.
[0041] Weigh BTC (60mg, 0.202mmol) into a 25mL two-necked bottle, add 5mL of anhydrous dichloromethane, stir magnetically, add equivalent N-methylaniline, triethylamine (0.25mmol) and Add 5mL of dichloromethane slowly in an ice bath, remove the ice-salt bath after the addition, and stir at room temperature for 4 hours.
[0042] Add HZA (40mg, 0.1mmol) to a 10ml single-necked bottle, add a trace of DMAP, 5mL of dichloromethane, ...
Embodiment 2
[0044] Embodiment 2: the synthesis (synthetic route figure such as figure 1 shown)
[0045]
[0046] The synthetic method of compound HZA is the same as above.
[0047] Weigh BTC (58mg, 0.2mmol) into a 25mL two-necked bottle, add 5mL of anhydrous dichloromethane, stir magnetically, add an equivalent amount of N-ethylaniline, triethylamine (0.25mmol) and Add 5mL of dichloromethane slowly in an ice bath, remove the ice-salt bath after the addition, and stir at room temperature for 4 hours.
[0048] Add HZA (40mg, 0.1mmol) to a 10ml single-necked bottle, add a trace of DMAP, 5mL of dichloromethane, and stir magnetically at room temperature until completely dissolved. Slowly add the solution dropwise to the reaction solution in the previous step under the ice-salt bath, remove the ice-salt bath after the dropwise addition, and stir at room temperature. After the reaction is completed, add 3 to 6 times the volume of distilled water to the above reaction solution, then extract...
Embodiment 3
[0050] Embodiment 3: the synthesis (synthetic route figure such as figure 1 shown)
[0051]
[0052] The synthetic method of compound HZA is the same as above.
[0053] Weigh BTC (58mg, 0.2mmol) into a 25mL two-necked bottle, add 5mL of anhydrous dichloromethane, stir magnetically, add an equivalent amount of N-methyl-o-methylaniline, triethylamine (0.25 mmol) and 5mL of dichloromethane were slowly added dropwise in an ice bath, and the ice-salt bath was removed after the dropwise addition, and stirred at room temperature for 4h.
[0054] Add HZA (40mg, 0.1mmol) to a 10ml single-necked bottle, add a trace of DMAP, 5mL of dichloromethane, and stir magnetically at room temperature until completely dissolved. Slowly add the solution dropwise to the reaction solution in the previous step under the ice-salt bath, remove the ice-salt bath after the dropwise addition, and stir at room temperature. After the reaction is completed, add 3 to 6 times the volume of distilled water t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com