Preparation method of salvianolic acid Y
A technology of salvianolic acid and salvianolic acid, applied in the field of medicine and chemical industry, can solve the problems of low purity, waste of resources, high cost, etc., and achieve the effect of high purity, few steps and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: A kind of preparation method of salvianolic acid Y
[0053] Step 1, enrichment of salvianolic acid Y
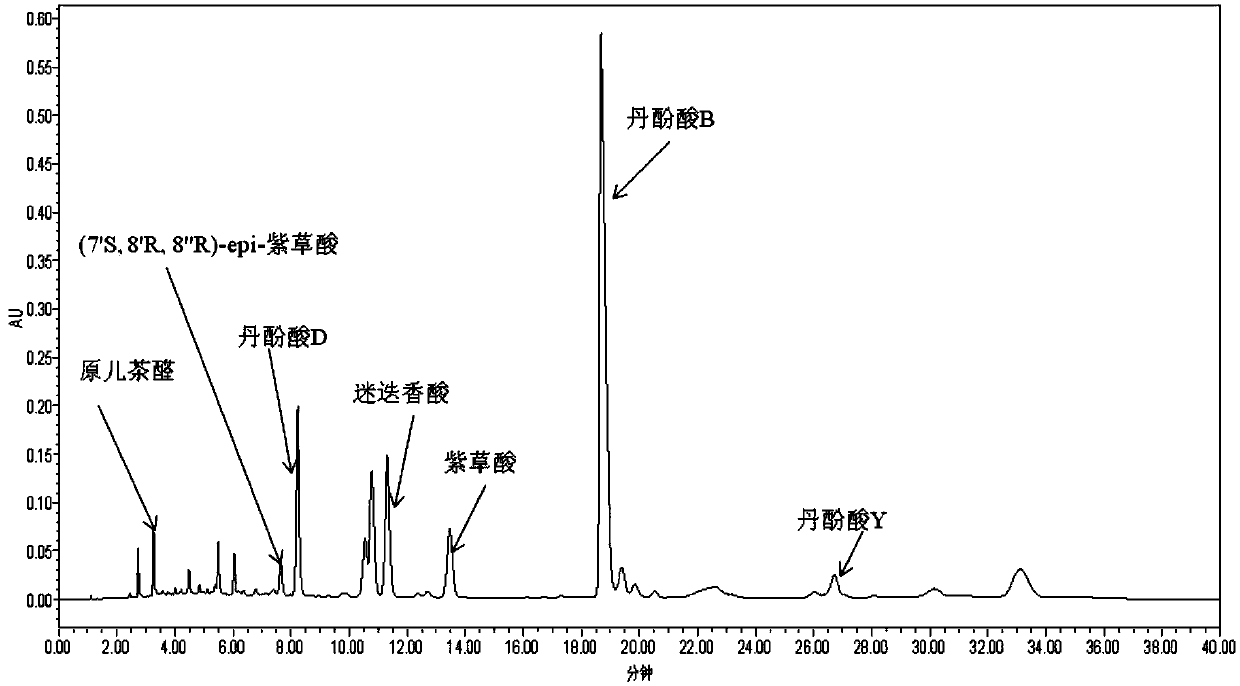
[0054] Take 500g of salvianolic acid extract, dissolve it in 1000ml of water (the weight-to-volume ratio of extract to water is 1:2), use a pH meter to detect the pH value is 5.5, load 100ml each time, add 3L MCI Gel glass In the chromatographic column (the ratio of extract mass to filler volume is 1:60), flow rate: 100ml / min. After sample loading, elute with 10% ethanol aqueous solution, flow rate: 100ml / min, take a sample every 300ml from the color of the bottom liquid, detect it by HPLC method, and compare it with the fingerprint of salvianolic acid extract, collect and combine Eluate containing salvianolic acid Y.
[0055] Adjust the pH value of the eluent to 3.0, add it again to a glass chromatographic column filled with 3LMCI Gel (the ratio of extract mass to filler volume is 1:6), wash 15L with water, discard the eluent, flow rate: 100ml / min. Th...
Embodiment 2
[0060] Embodiment 2: the enlarged preparation method of salvianolic acid Y
[0061] Step 1, enrichment of salvianolic acid Y
[0062] Take 1000g of salvianolic acid extract, dissolve it in 2000ml of water, use a pH meter to detect that the pH value is 5.5, load 100ml each time, add a 3L MCI Gel glass chromatographic column (the ratio of extract mass to filler volume is 1:60 ), flow rate: 100ml / min. After sample loading, elute with 10% ethanol aqueous solution, flow rate: 100ml / min, pick up a sample every 300ml from the color of the lower contact solution. Detected by HPLC method, and compared with the fingerprint of salvianolic acid extract at the same time, the eluate containing salvianolic acid Y in the 15th-25th bottle was combined.
[0063] Adjust the pH value of the eluent to 3.0, add it again to a 3L MCI Gel glass column (the ratio of extract mass to filler volume is 1:3), wash 15L with water, discard the eluent, flow rate: 100ml / min . Then elute with 95% ethanol, fl...
experiment example 1
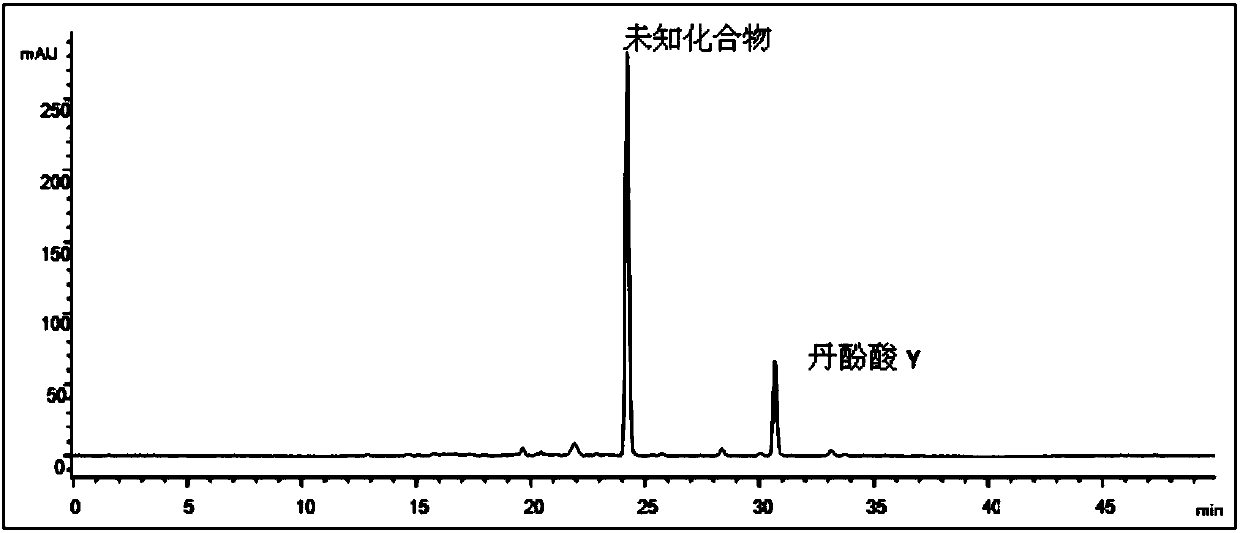
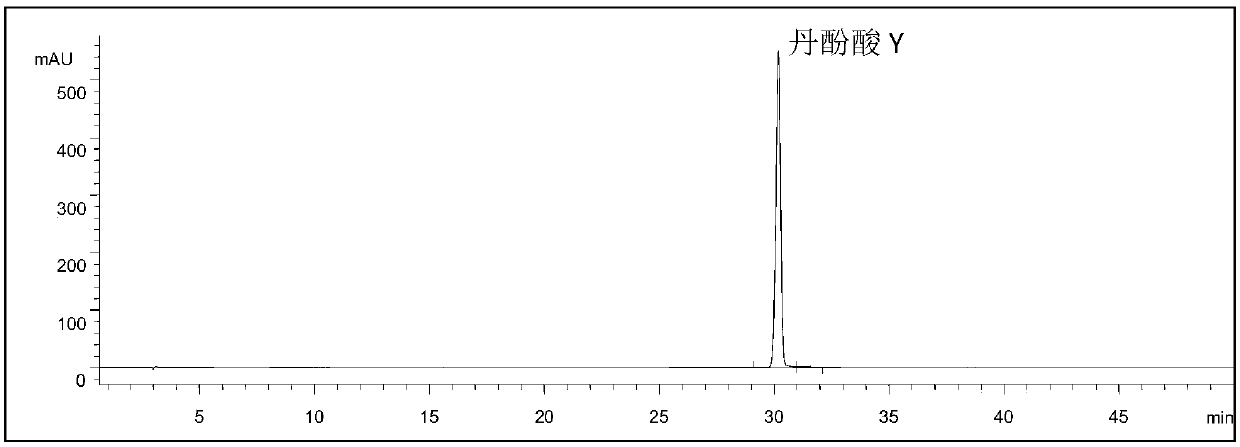
[0068] Experimental example 1: Determination of the content of salvianolic acid Y
[0069] 1. Using high performance liquid chromatography, the specific conditions are as follows
[0070] Instruments: Agilent 1100 high performance liquid chromatography, quaternary pump, UV detector.
[0071] Chromatographic column: Agilent C18 analytical column (4.6mm×250mm, 5μm); mobile phase: gradient elution, see Table 1; flow rate: 1mL·min-1; detection wavelength: 280nm; column temperature: 30°C.
[0072] Table 1 Gradient of chromatographic conditions
[0073] Solvent time (min) 0 8 15 35 40 50 A: 80% acetonitrile + 20% B 10% 22% 26% 39% 10% 10% B: 0.02% phosphoric acid aqueous solution 90% 78% 74% 61% 90% 90%
[0074] 2. Preparation of the sample solution to be tested: Take 10 mg of salvianolic acid Y, weigh it accurately, put it in a 25ml volumetric flask, dissolve it with methanol, and set the solution to the mark, and wait for the test.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com