Method for preparing apixaban
A technology of apixaban and compounds, which is applied in the field of preparation of pharmaceutical compounds, can solve problems such as long routes, unsuitable for industrial production of raw materials, expensive metal catalysts, etc., achieve high reaction yield, facilitate industrial production, and facilitate simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
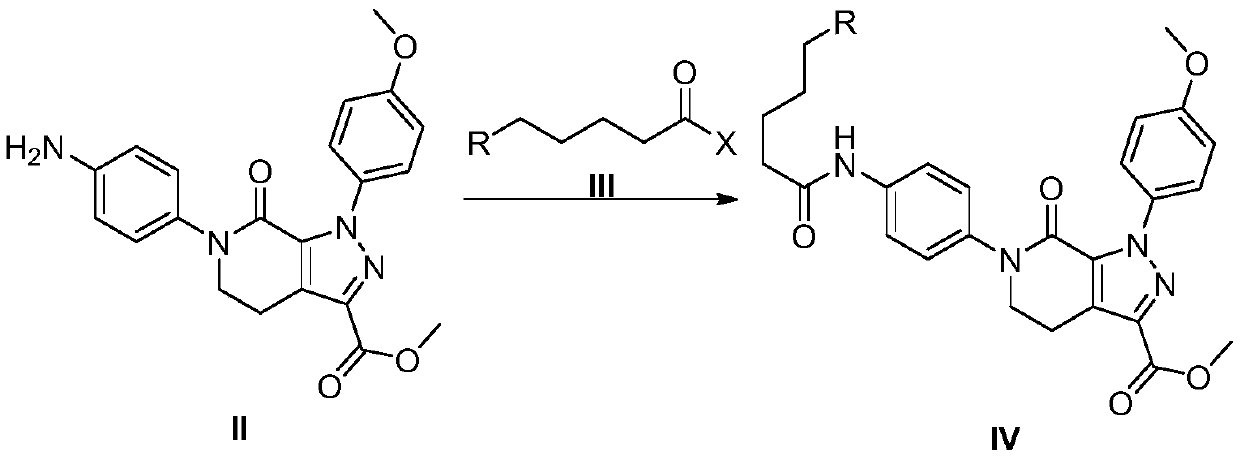
[0062]
[0063] At -5°C to 0°C, add 80Kg of acetone, compound II (10.0Kg, 25.5mol), and 40% potassium carbonate aqueous solution (10Kg, 29.0mol) into the reaction kettle (300L). Stir to lower the temperature, control the temperature from -5°C to 0°C, and slowly add 5-chlorovaleryl chloride (4.7Kg, 30.3mol). The reaction was incubated for 30 minutes, and TLC detected that there was no residue of the raw material compound II. Add 120Kg of purified water, and control the temperature at -5°C to 0°C to stir and crystallize for 20min. centrifugal. Dry at 50°C to obtain 12.4Kg of compound IV.
[0064] Yield: 97.1%, HPLC: 99.71%.
Embodiment 2
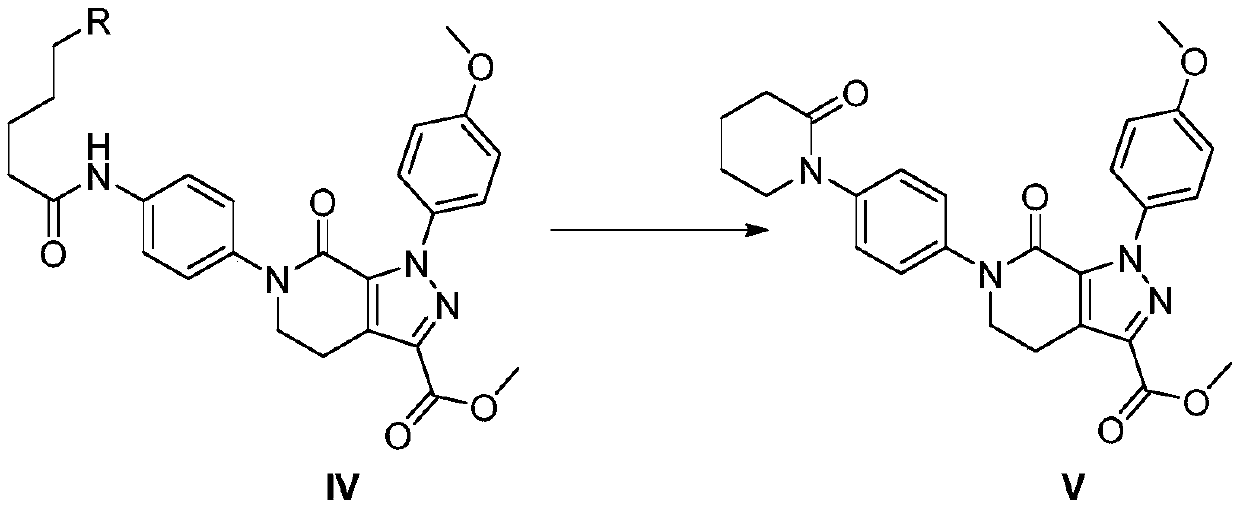
[0066]
[0067] At 25°C to 30°C, add 133Kg of dichloromethane, compound IV (10.0Kg, 19.6mol), potassium carbonate (7.2Kg, 52.1mol), tetrabutylammonium fluoride (6.7Kg, 25.6 mol). Stir the reaction for 1 h, and TLC detects that there is no residue of the starting compound IV. Add 100kg of purified water, stir and let stand to separate layers, continue to wash the organic phase once with 100kg of water, and dry with 5kg of anhydrous sodium sulfate for 1 hour. After filtering, the filtrate was concentrated, and after no effluent was added, 70 kg of isopropyl acetate was added, stirred for 2 hours, and centrifuged. Dry at 50°C to obtain 7.5Kg of compound V.
[0068] Yield: 80.6%, HPLC: 99.45%.
Embodiment 3
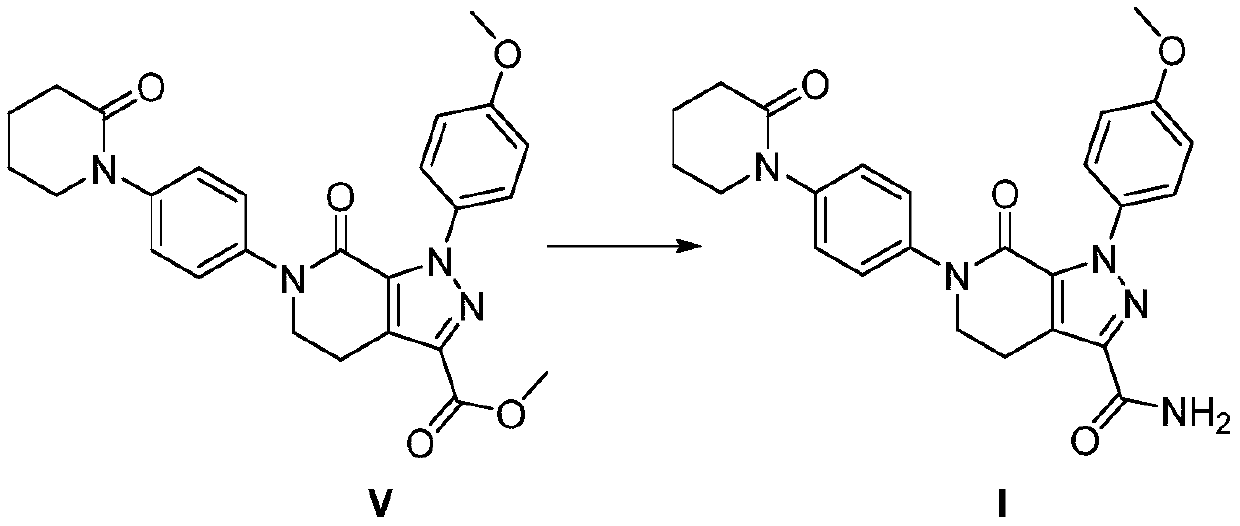
[0070]
[0071] At -5°C to 5°C, add 27Kg of N,N-dimethylformamide, compound V (7.0Kg, 14.7mol) and formamide (6.7Kg, 148.8mol) into the reaction kettle (100L). Stir to lower the temperature, control the temperature at -5°C to 5°C, and slowly add 30% sodium methoxide methanol solution (3.7Kg, 20.6mol). The reaction was incubated for 1 h, and no residual compound V was detected by TLC. Continue to insulate and stir for 2h. centrifugal. The solid was transferred to a reaction kettle (100 L), and 50 kg of ethanol was added for beating for 1 hour, and centrifuged. Dry at 50°C to obtain 5.4Kg of compound I.
[0072] Yield: 79.9%, HPLC: 99.89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com