Nickel phosphide catalyst used for producing 2,3,3,3-tetrafluoropropene through gas-phase selective hydrodechlorination
A technology for selective hydrogenation and tetrafluoropropene, which is used in physical/chemical process catalysts, dehydrohalogenation preparation, organic chemistry, etc. It can solve the problems of reduced selectivity of target products and increased cost of reaction processes, and achieve high temperature sintering resistance. Good, low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
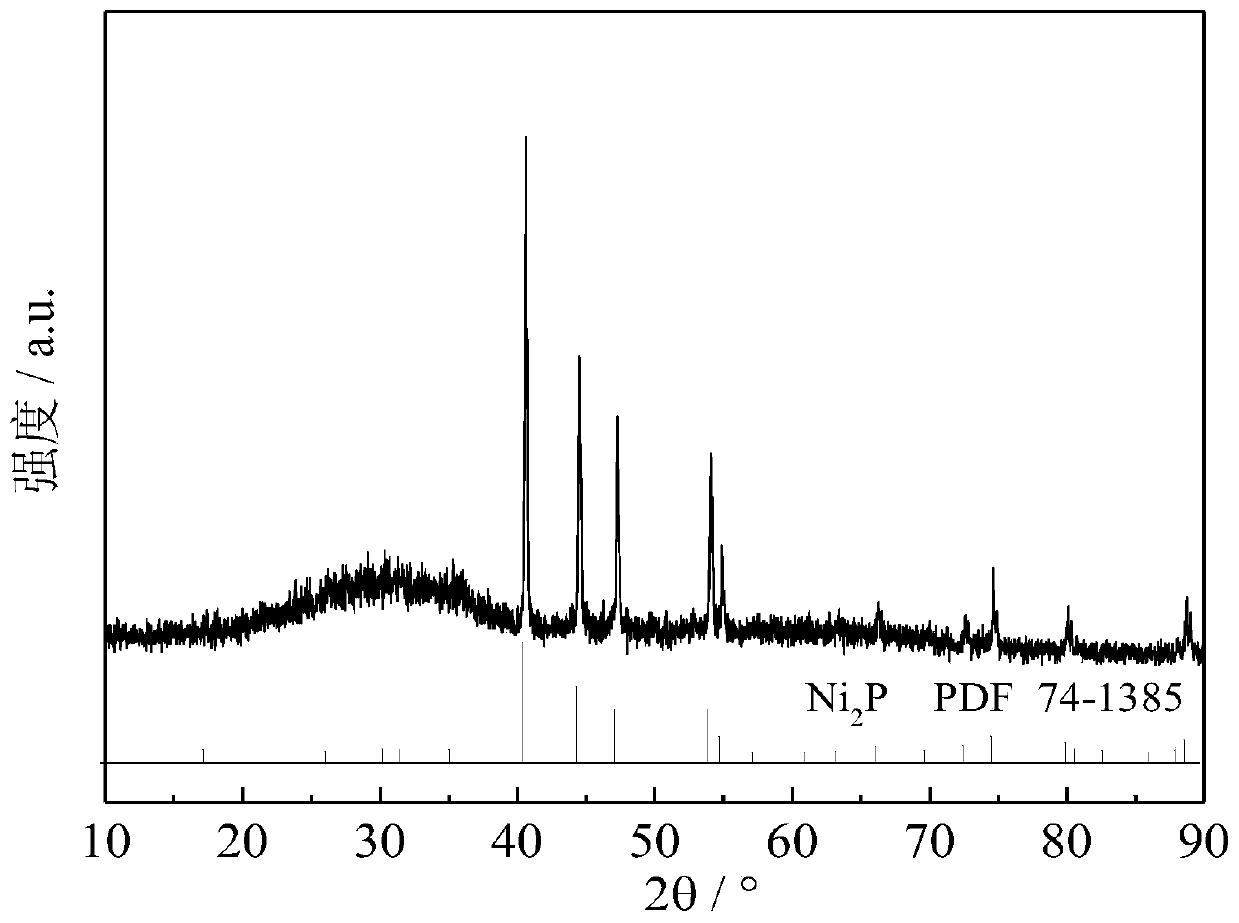
[0022] Embodiment 1: prepare Ni 2 P / oxide (fluoride, molecular sieve) catalyst
[0023] 9.48g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O) and 3.45g diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) was added to 20 mL of deionized aqueous solution, and then the pH of the solution was adjusted to 2-3 with concentrated nitric acid to obtain a clear green solution. According to the loading of 0.5%, 5%, 10%, 20%, and 30%, the above solutions are impregnated onto a certain mass of oxides, fluorides, or molecular sieves, and then aged at room temperature for 12 hours, dried at 120 ° C for 12 hours to dry the water, and Calcined at 500°C for 3h to obtain the precursor of phosphate. The phosphide catalyst was prepared by in-situ temperature-programmed reduction method. The temperature programming step mainly includes two steps: (1) in H 2 Under the atmosphere (flow rate 150mL / min), the temperature was raised from room temperature to 120°C at 5°C / min, and kept at 120°C for 1h to remove...
Embodiment 2
[0024] Embodiment 2: prepare Ni 12 P 5 / activated carbon catalyst
[0025] 9.48g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O) and 1.79g diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) was added to 20 mL of deionized aqueous solution, and then the pH of the solution was adjusted to 2-3 with concentrated nitric acid to obtain a clear green solution. According to the loads of 5%, 10%, 20%, and 30%, the above solutions were impregnated on a certain mass of activated carbon, and then aged at room temperature for 12 hours, dried at 120 ° C for 12 h, and roasted at 500 ° C for 3 h in a nitrogen atmosphere to obtain Phosphate precursor. The phosphide catalyst was prepared by in-situ temperature-programmed reduction method. The temperature programming step mainly includes two steps: (1) in H 2 Under the atmosphere (flow rate 150mL / min), the temperature was raised from room temperature to 120°C at 5°C / min, and kept at 120°C for 1h to remove the water adsorbed by the catalyst; (2) fro...
Embodiment 3
[0026] Example 3: Using the catalyst prepared by the method in Example 1 and 2, apply it to the gas phase selective hydrodechlorination of 2-chloro-1,1,1,2-tetrafluoropropane to prepare 2,3,3,3- In the reaction of tetrafluoropropene, after running for 8 hours, the reaction results are as follows:
[0027] Table 1 Conversion rate and product selectivity of HCFC-244bb on nickel phosphide catalysts with different loadings on different carriers for selective hydrodechlorination reaction.
[0028] Numbering catalyst HCFC-244bb conversion rate / % HFO-1234yf selectivity / % 1 0.5wt.%Ni 2 P / SiO 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com