Novel formulations of amidine substituted beta-lactam compounds on the basis of modified cyclodextrins and acidifying agents, their preparation and use as antimicrobial pharmaceutical compositions
A compound and cyclodextrin technology, applied in the field of antimicrobial pharmaceutical compositions, can solve the problems of inability to provide drug loading and stability, and achieve the effect of enhancing aqueous stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0383] Example 1 - Preparation of Formulation Solutions on Laboratory Scale
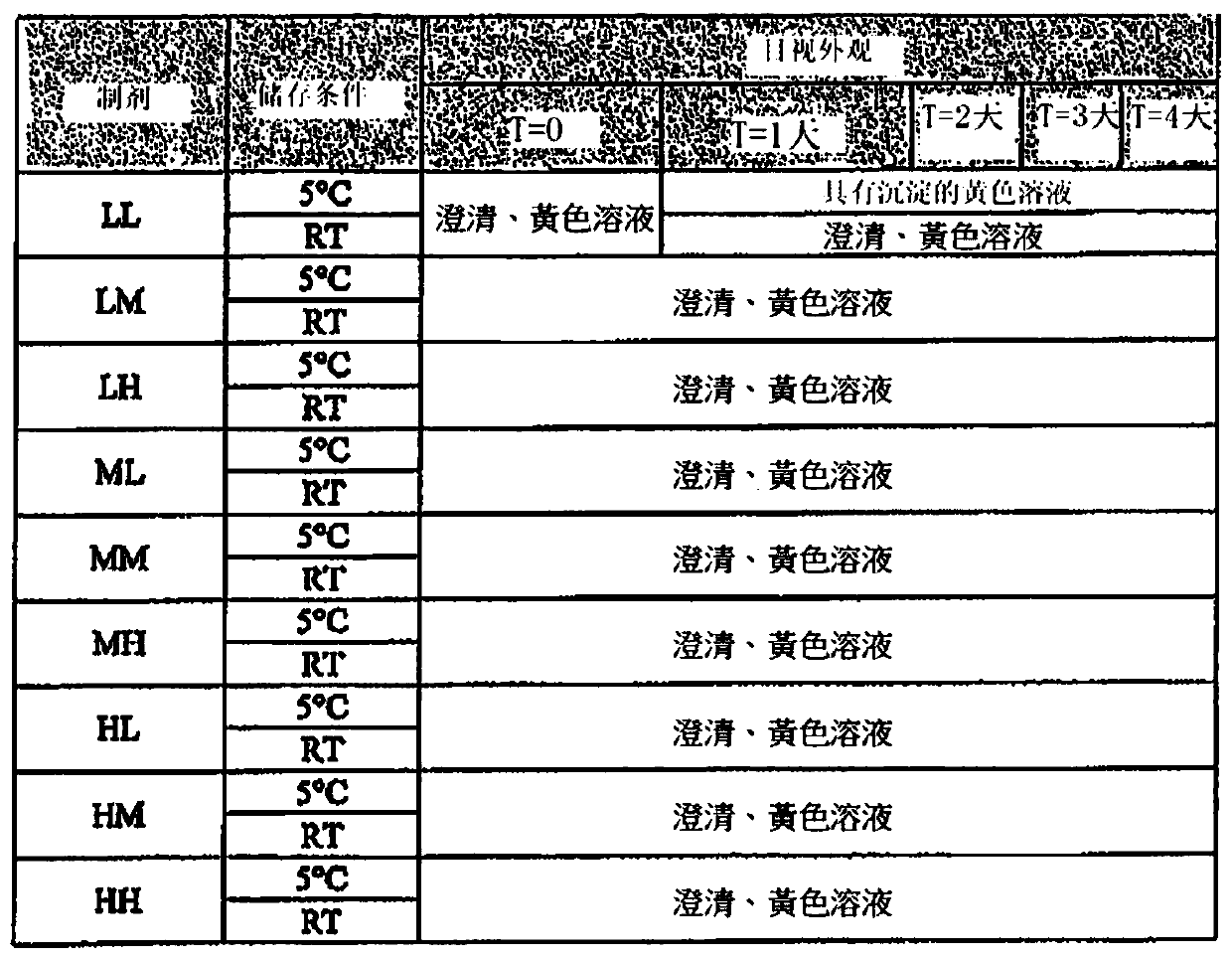
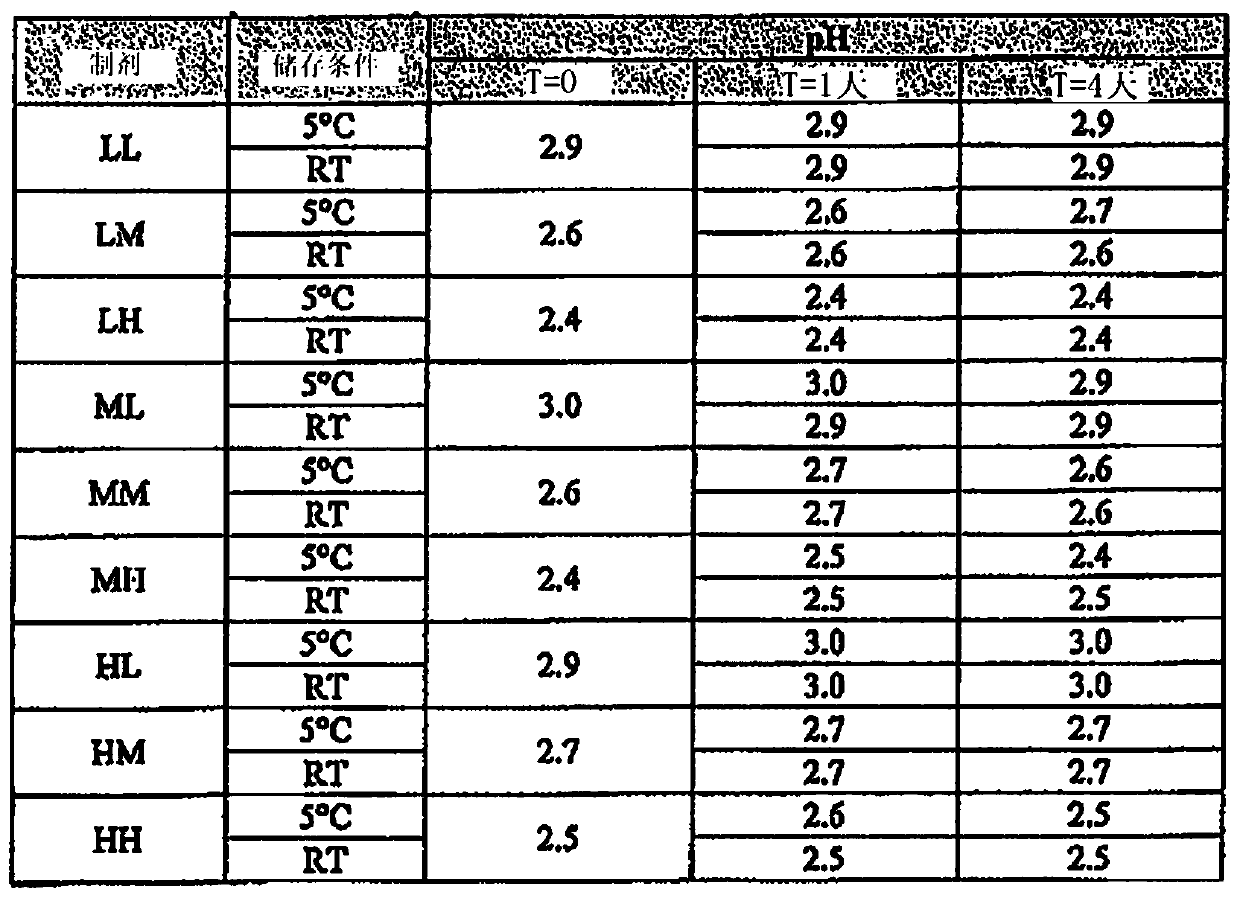
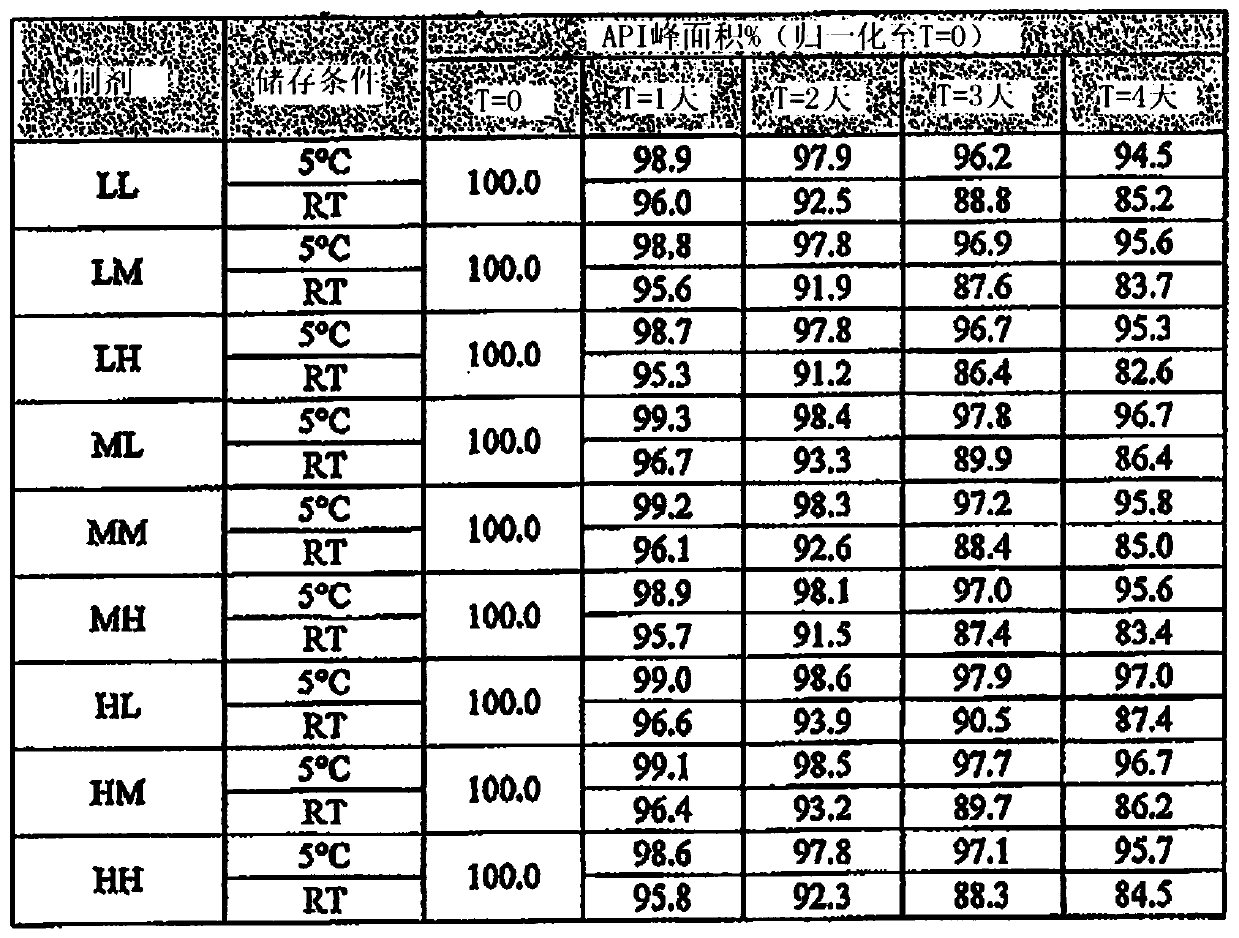
[0384] For the SBE-β-CD / CA formulations of the present invention, nine different exemplary formulations were tested as outlined below. These formulations had high, medium or low levels of each of the two excipients, SBE-β-CD and CA. The nominal formulation has moderate levels of SBE-β-CD and CA. High or low levels of SBE-β-CD were defined as plus or minus 40% of its nominal concentration, respectively. High or low levels of citric acid were defined as 1.5% CA plus or minus 3.5% of its nominal concentration, respectively.
[0385] An exemplary SBE-β-CD / CA placebo formulation solution was prepared by directly adding the compound of formula (I) as the API to the solution, followed by warming the solution in a 50°C water bath so that the solution was shaken. At the same time dissolve the API.
[0386] These placebo solutions were prepared in 9 different combinations, abbreviated as LL, LM, LH, ML, MM...
example 2
[0410] Example 2 – Scale Stability
[0411] In this study on the stability of Compound (I) as an API in formulations of the invention, 50 mL batches of the exemplary API in 20% SBE-β-CD and 1% CA have been tested in up to 12 Photostability and storage stability within one month.
[0412] Therefore, experiments have been performed to evaluate critical quality attributes of pharmaceutical products (ie compounds of formula (I)) under different storage conditions.
[0413] Example 2 summarizes the photostability and test results of the stability study at the following time points: time 0 (start), 1 month, 2 months, 3 months, 6 months, 9 months and 12 months.
[0414] batch production
[0415] A 3L batch (number WO 2015-0213) was manufactured at Lake Forest on June 15, 2015. The formulations were mixed and filtered in the laboratory (see formulation in Table 3); and filled and lyophilized at the pilot plant. The target fill volume was 15.6 mL / vial. Vials were half-packed and s...
example 3
[0461] Example 3 - Solubility of Compounds of Formula (I) as API in SBE-β-CD
[0462] The solubility of the compound of formula (I) as API is highly pH dependent, as Figure 23 shown in .
[0463] In the concentration range of 0 to 20% SBE-β-CD, the corresponding phase solubility curve appears to be linear, that is, A L type. Deviations may be due to pH fluctuations (see Figure 24 ).
[0464] Such as Figure 24 A shown in L The phase-solubility curve indicates the formation of Compound (I)-SBE-β-CD 1:1 complex, ie one molecule of Compound (I) forms a complex with one molecule of SBE-β-CD. Therefore, the intrinsic solubility (i.e., the solubility in the absence of SBE-β-CD or S 0 ) and the slope of the linear curve to estimate the stability constant of the complex (K 1:1 ):
[0465]
[0466] The solubility properties of the API of the compound of formula (I) were measured at two different pH values (see Figure 25 ):
[0467] At pH 4.0:
[0468] At pH 7.4: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com