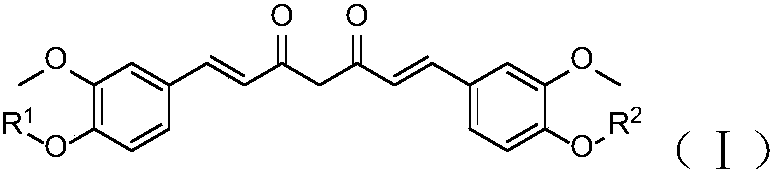
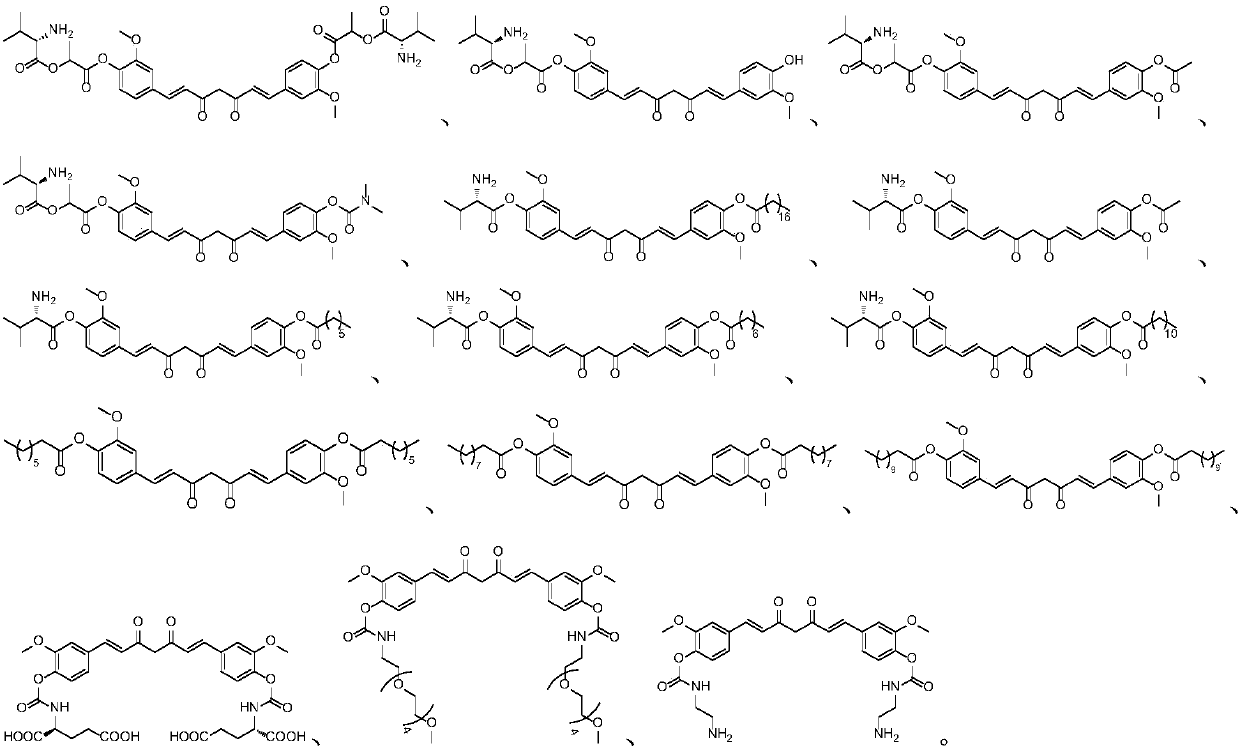
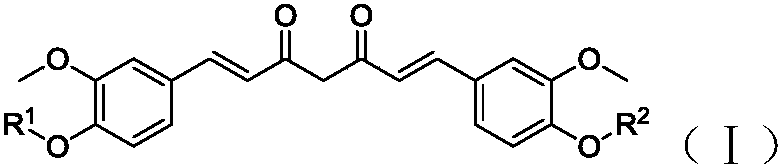
Curcumin derivative and uses thereof
A halogen and compound technology, applied in the field of medicinal chemistry, can solve problems such as short plasma half-life, poor stability of curcumin, and difficult oral absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of Compound 1
[0053]
[0054] Preparation of intermediate 1-1 Boc-L-valine benzyl lactate
[0055] Under ice-bath conditions, 27.00g (150mmol) of benzyl lactate, 32.54g (150mmol) of Boc-valine, and 400mL of dichloromethane (DCM) were added to the three-necked reaction flask, and then 61.85g (300mmol) of DCC were added in batches Adding to the system, the adding speed is to control the system not to reflux. Then the temperature was naturally raised to room temperature, and the reaction was carried out until the benzyl lactate was completely converted. Stop the reaction, filter the reaction solution, spin the filtrate to dryness, then add 100mL diethyl ether to freeze in the refrigerator for more than 2 hours, then filter, dilute the filtrate with 400mL DCM, wash with ice-cold 200mL 1N sodium hydroxide, 200mL saturated saline, and then separate to obtain a DCM solution , dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-...
Embodiment 2
[0064] Example 2 Preparation of Compound 2
[0065]
[0066] Preparation of Intermediate 2-1 Acetyl Curcumin
[0067] Under ice-bath condition, curcumin 20.00g (54.28mmol), 21.46g pyridine (271.40mmol), 0.66gDMAP (5.40mmol) and 400mL DCM are added in the three-port reactor, then will contain acetic anhydride 5.54g (54.28mmol) DCM (40 mL) solution was added dropwise into the system, and the dropwise addition was completed in about 10 minutes. Then naturally warm up to room temperature and react for 2 hours. The reaction was stopped, and the reaction solution was washed with 200 mL of 1N hydrochloric acid and 200 mL of water, separated to obtain a DCM solution, dried over anhydrous sodium sulfate, and spin-dried to obtain a crude product. A yellow solid product (7.60 g, 34.1%) was obtained by silica gel column chromatography.
[0068] Preparation of intermediate 2-2 Boc-L-valine-lactic acid-acetyl curcumin
[0069] Acetyl curcumin 3.00g (7.30mmol), Boc-valyl lactic acid 2....
Embodiment 3
[0074] Example 3 Preparation of Compound 3
[0075]
[0076] Preparation of intermediate 3-1 curcumin monostearate
[0077] Under ice-bath condition, curcumin 10g (27.14mmol), 10.73g pyridine (135.70mmol), 0.33g DMAP (2.70mmol) and 200ml DCM are added in the three-necked reaction flask, then will contain stearic acid 5.81g (21.52mmol) 20mL of DCM solution was added dropwise into the system, and the dropwise addition was completed in about 10 minutes. Then naturally warm up to room temperature and react for 2 hours. The reaction was stopped, and the reaction solution was washed with 100 mL of 1N hydrochloric acid and 100 mL of water, separated to obtain a DCM solution, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated to obtain a crude product, and silica gel was used to obtain a yellow solid product (4.20 g, 31.3%).
[0078] Intermediate 3-2 Preparation of Boc-L-valine-stearic acid-curcumin
[0079] Curcumin monostearate 3g (6.06mmol), Boc-vali...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com