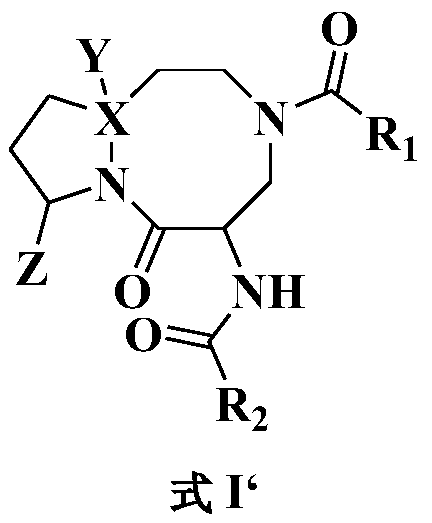
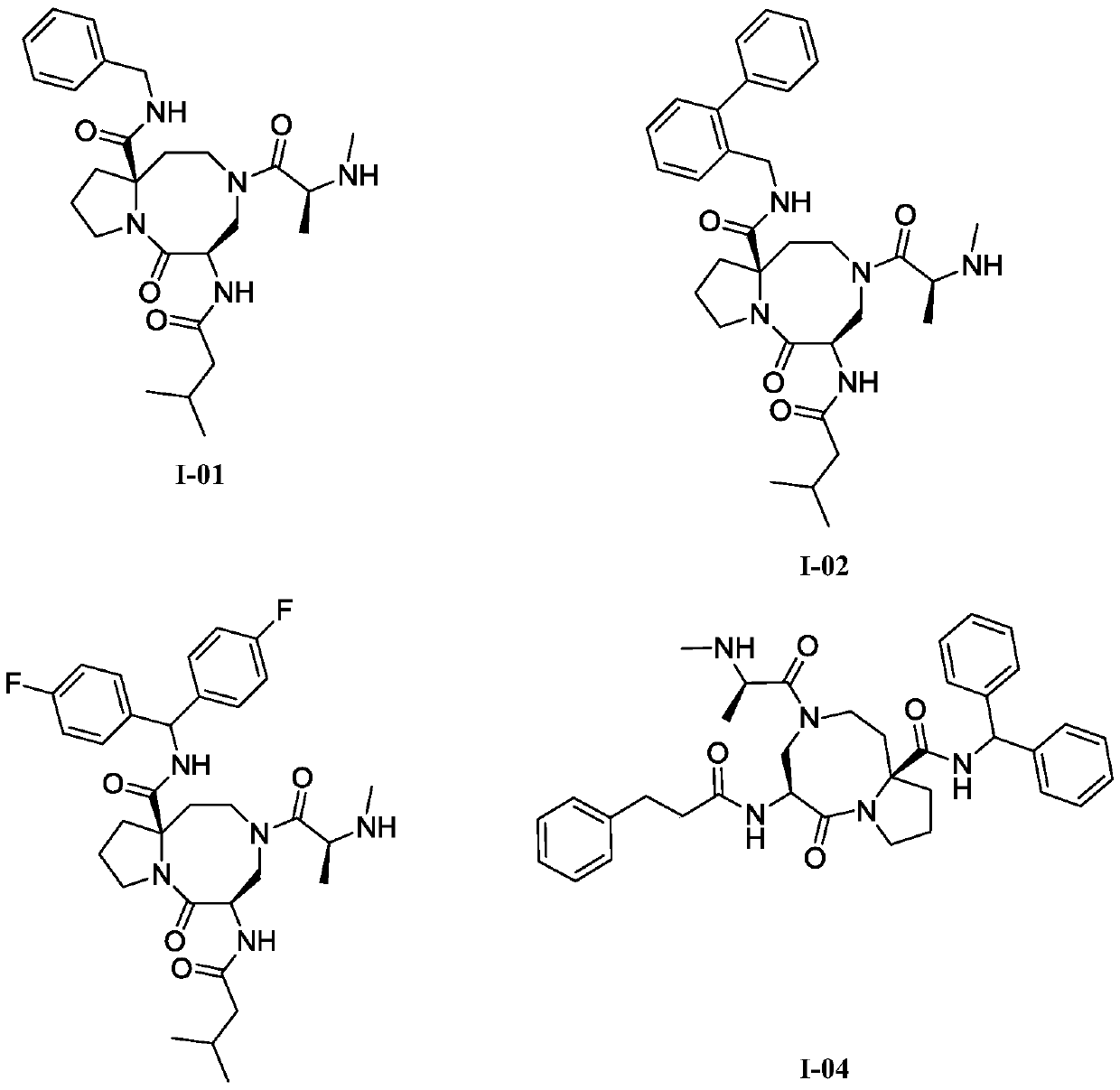
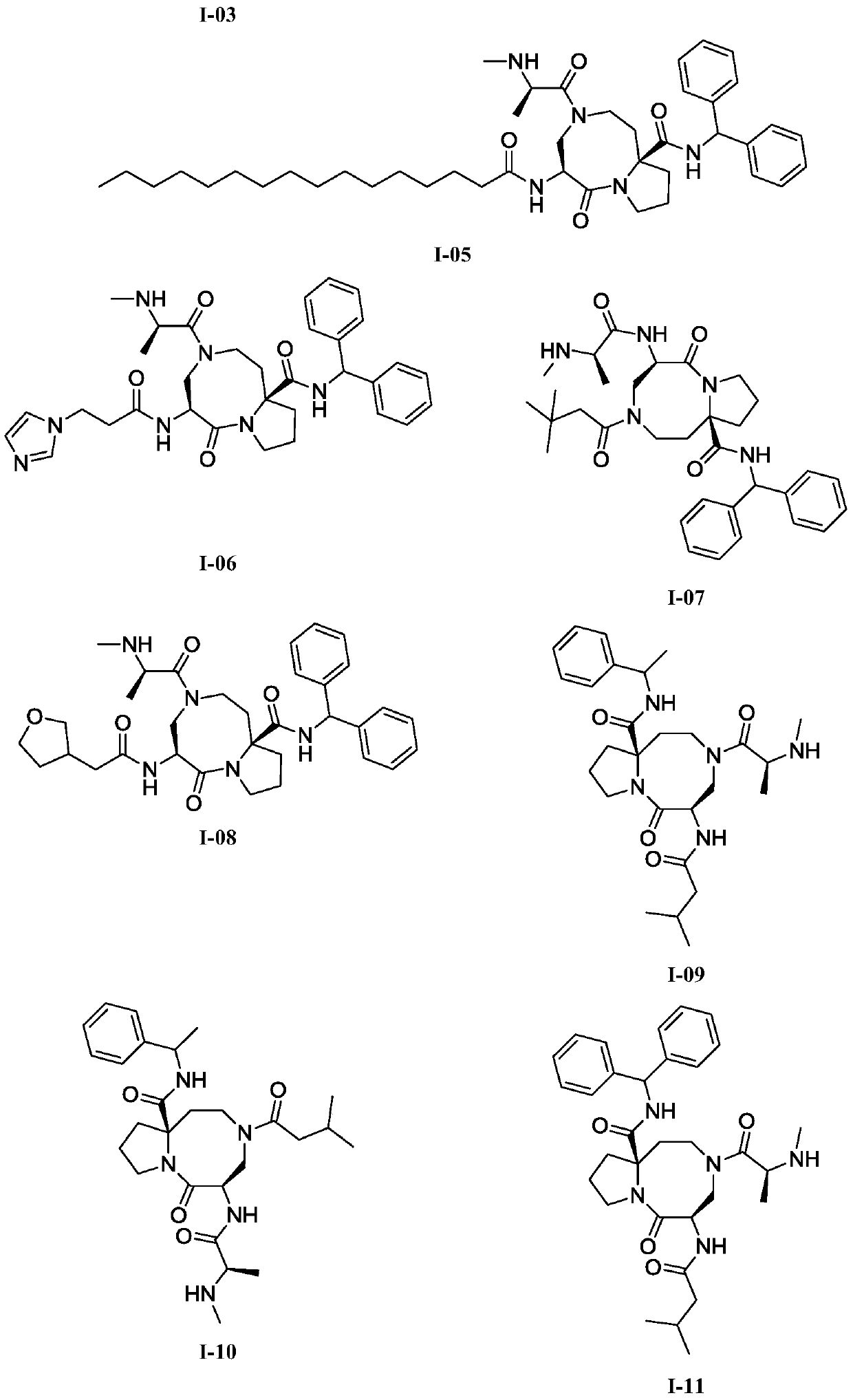
Anti-tumor diazo dicyclic apoptosis protein inhibitor
A technology of cycloalkylamine group and compound, applied in the field of medicinal chemistry, can solve the problems of weak effect on solid tumors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1: the preparation of pyrrolidine-2-methyl carboxylate
[0094]
[0095] Take 40g (0.35mol) of proline and 250mL of methanol in a 500mL single-port reaction flask, cool to 0°C, take another 45g (0.38mol) of thionyl chloride in a 250mL constant pressure dropping funnel, and slowly add it dropwise to the above In the solution system, the system releases heat with the dropwise addition, and the internal temperature is controlled to be <5°C, and the dropwise addition is completed in about 20 minutes. The temperature was raised to 70°C to obtain a clear solution, which was refluxed for 3 hours. After cooling down to room temperature, the solvent was distilled off under reduced pressure to obtain 43 g of light yellow oily liquid, which was directly used in the next reaction.
Embodiment 2
[0096] Embodiment 2: the preparation of N-Boc pyrrolidine-2-methyl carboxylate
[0097]
[0098] Take 43g (0.33mol) of methyl pyrrolidine-2-carboxylate and 400mL of dichloromethane in a 2L single-port reaction flask, add 88g (0.87mol) of triethylamine to obtain a white emulsion, stir at room temperature for 15 minutes, and cool to 0°C.
[0099] Another 152g (0.69mol) of Boc anhydride was dissolved in 600mL of dichloromethane in a constant pressure funnel, and added dropwise to the above system. A large number of bubbles were released along with the dropwise addition, exothermic, and the addition rate was controlled to maintain the internal temperature of the system <10°C. After the dropwise addition, a white suspension was obtained, which was stirred at room temperature for 12 hours. Filter to remove the white precipitate, evaporate the solvent from the filtrate under reduced pressure to obtain a white suspension, add 300mL citric acid solution (0.5M) to wash, extract with...
Embodiment 3
[0100] Embodiment 3: Preparation of N-Boc-2-propenyl-pyrrolidine-2-carboxylic acid methyl ester
[0101]
[0102] Take 20 g (87.3 mmol) of methyl N-Boc pyrrolidine-2-carboxylate and 200 mL of tetrahydrofuran in a 500 mL three-neck flask, protect with nitrogen, and cool to -78°C.
[0103] Another 105mL (105mmol, 1M) of lithium bistrimethylsilylamide was put into the constant pressure dropping funnel, and added dropwise to the above system, and the dropping rate was controlled to keep the internal temperature of the system <-78°C to obtain a yellow-brown solution. After the dropwise addition was completed, the mixture was stirred at -78°C for 1.5 hours.
[0104] Another 15.9 g (131 mmol) of allyl bromide was dissolved in 100 mL of tetrahydrofuran, added dropwise to the above system, and the rate of addition was controlled to maintain the internal temperature of the system < -78°C. After the addition was complete, stir at -78°C for 1 hour. Return to room temperature and stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com