Preparation method of tosufloxacin tosylate monohydrate
A technology of tosufloxacin tosylate and monohydrate, which is applied in the field of drug synthesis, can solve the problems of large amount of waste water and liquid, poor product quality, and low yield, so as to reduce the discharge of "three wastes" and shorten the production cycle , the effect of improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
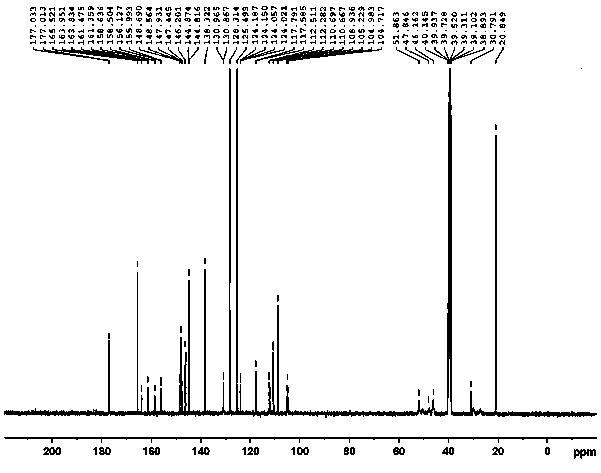
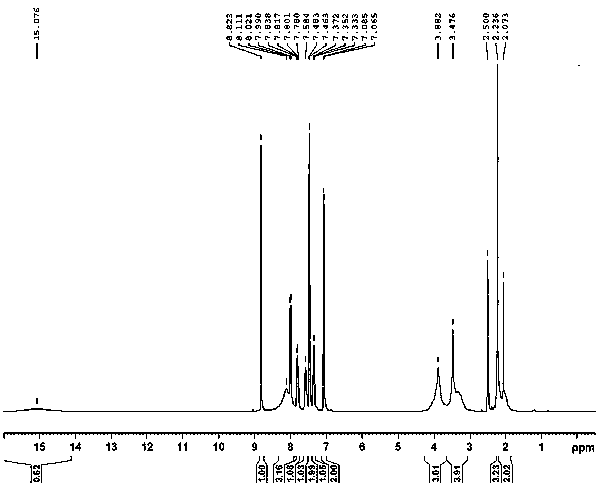
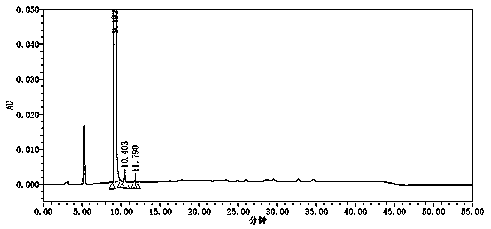
[0033] A preparation method of tosufloxacin tosylate monohydrate, adding 2000ml of acetone and 2500.0ml of water into a 10L reaction kettle for mixing, and then sequentially adding 500g of 7-(3-aminopyrrolidin-1-yl) -1-(2,4-difluorophenyl)-6-fluoro-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid ethyl ester (formula Ⅱ), p Toluenesulfonic acid monohydrate 901.83g, mechanically stirred and heated, the temperature of the system was controlled at 65~70°C, stirred and reacted for 6 hours, the residual amount of raw materials was detected by sampling HPLC ≤ 0.5%, 50g of activated carbon was added to the system, and continued at 65~70°C Stir for 0.5h, filter while hot, stir and cool the filtrate to 0-5°C, keep stirring and crystallize for 7 hours. Filter and wash the filter cake with 1000ml of purified water; dry the filter cake at 55~65°C for 8~18 hours to obtain 611.9g of off-white tosufloxacin tosylate monohydrate (Formula Ⅰ), with a yield of 89.0% , 99.90% purity.
[003...
Embodiment 2
[0042] Add 101.3ml of ethanol and 100.0ml of water into the reaction flask for mixing, then add 20.0 g of 7-(3-aminopyrrolidin-1-yl)-1-(2,4-difluorophenyl)-6- Fluoro-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid ethyl ester (Formula II) and p-toluenesulfonic acid monohydrate 36.0g, stirred and heated, the system temperature was controlled at 65 ~85°C, keep stirring and reflux for 7 hours, sample HPLC to detect the residual amount of raw materials is ≤0.5%, add 2.0g of activated carbon to the system, continue stirring for 0.5 hours at 65~70°C, filter while hot, and cool the filtrate to 0~5 ℃, heat preservation and crystallization for 4h. Filter and wash the filter cake with 40.0ml of purified water; dry the filter cake at 55-65°C for 8-18 hours to obtain 25.1 g of off-white tosufloxacin tosylate monohydrate (Formula Ⅰ), with a yield of 91.2 %, purity 99.42%
Embodiment 3
[0044] Add 126.6ml methanol and 100.0ml water into the reaction flask for mixing, then add 20.0 g of 7-(3-aminopyrrolidin-1-yl)-1-(2,4-difluorophenyl)-6- Add ethyl fluoro-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylate (Formula II) and p-toluenesulfonic acid monohydrate 36.0g into the reaction flask in turn, stir and heat , the temperature of the system is controlled at 65~70°C, heat-preserved, stirred and refluxed for 7 hours. Sampling HPLC detects that the residual amount of raw materials is ≤0.5%. Add 2.0g of activated carbon to the system, continue to stir at 65~70°C for 0.5h, filter while it is hot, and stir the filtrate Cool down to 20~25°C, keep warm and crystallize for 4h. Filter and wash the filter cake with 40.0ml of purified water; dry the filter cake at 55~65°C for 8~18 hours to obtain 24.9g of off-white tosufloxacin tosylate monohydrate (Formula Ⅰ), with a yield of 90.5 %, purity 99.47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com