Triazin ring containing covalent organic microporous polymer and application method and application thereof
A microporous polymer, covalent organic technology, applied in the direction of separation methods, chemical instruments and methods, educts, etc., can solve the problems of difficult recycling, difficult regeneration, strong corrosion, volatile and easy decomposition, etc., to increase The contact area and the effect of improving the adsorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The preparation of poly(2,4,6-three (p-cyanoaniline)-1,3,5-triazine) comprises the following steps:
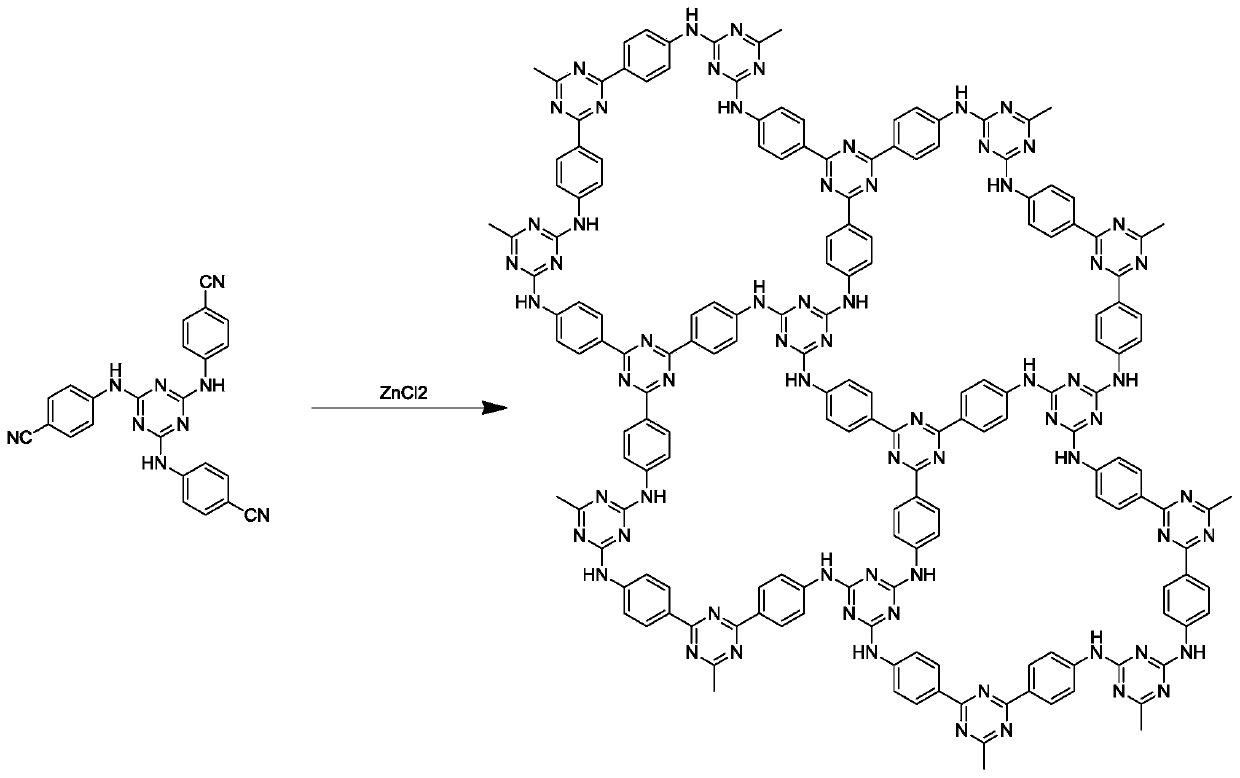
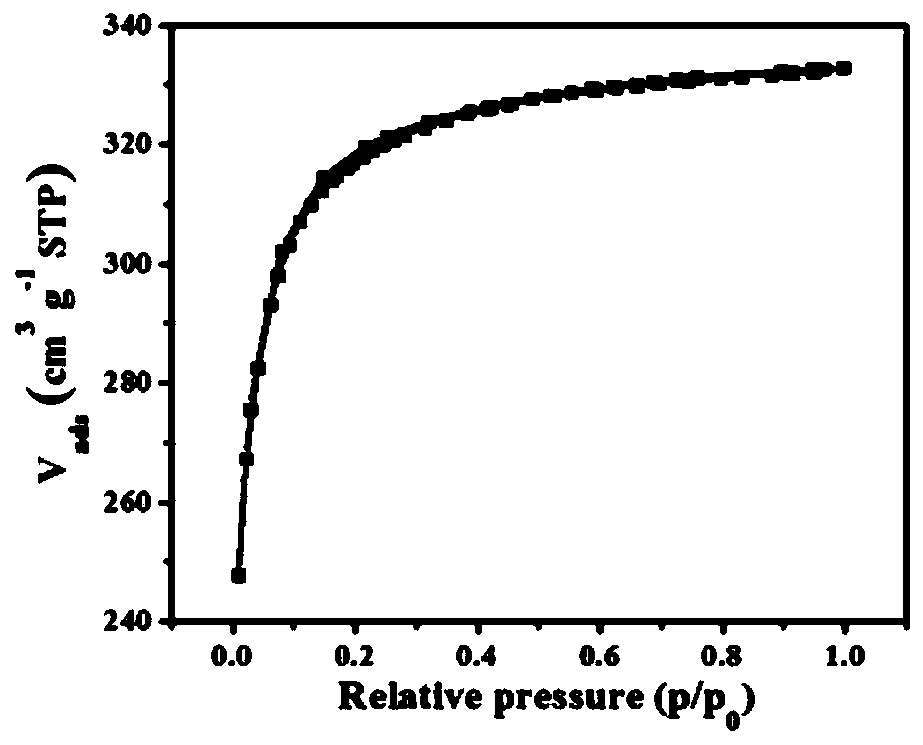
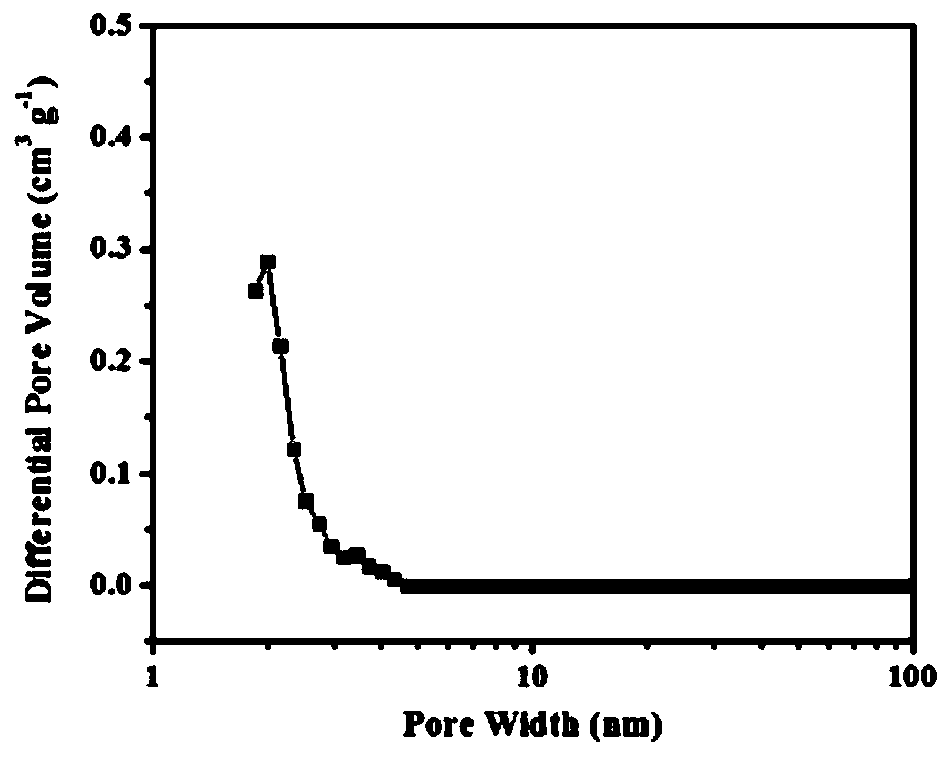
[0066] Add 429mg (1mmol) 2,4,6-tris(p-cyanoaniline)-1,3,5-triazine, 613.4mg (5mmol) zinc chloride to the sealed tube and grind with a mortar under infrared lamp Evenly, then put into the sealed tube. After the tube was sealed, it was placed in a muffle furnace at 600°C for 40 hours of reaction to obtain a polymer. After the reaction is complete, put the polymer in a 500ml flask, add 200ml of distilled water, ultrasonicate for 1h, then wash at 90°C for 12h under stirring, filter, then stir in 0.1M dilute hydrochloric acid for 12h, filter, wash with water until neutral, and then use Washing with dimethyl sulfoxide and acetone, the polymer is obtained after vacuum drying, and its synthetic route is as follows: figure 1 Shown; its nitrogen adsorption-desorption curve and pore structure distribution are as follows figure 2 and image 3 Shown; its carbon dioxide adsorpti...
Embodiment 2
[0068] The preparation of poly(2,4,6-three (p-cyanoaniline)-1,3,5-triazine) comprises the following steps:
[0069] Add 429mg (1mmol) 2,4,6-tris(p-cyanoaniline)-1,3,5-triazine, 613.4mg (5mmol) zinc chloride to the sealed tube and grind with a mortar under infrared lamp Evenly, then put into the sealed tube. After sealing the tube, place it in a muffle furnace for reaction. The process is: react at 250°C for 15h, then react at 300°C for 10h, then react at 350°C for 10h, and finally react at 400°C for 25h, a total of 60h, to obtain a polymer. Put the polymer in a 500ml flask, add 200ml of distilled water, ultrasonicate for 1h, then wash at 90°C for 12h with stirring, filter, then stir in 0.1M dilute hydrochloric acid for 12h, filter, wash with water until neutral, and then wash with dimethyl After washing with sulfoxide and acetone, the polymer is obtained after vacuum drying, and its synthetic route is as follows figure 1 Shown; its nitrogen adsorption-desorption curve and po...
Embodiment 3
[0071] The preparation of poly(2,4,6-three (p-cyanophenoxy)-1,3,5 triazine) comprises the following steps:
[0072]Add 432 mg (1 mmol) 2,4,6-tris(p-cyanophenoxy)-1,3,5 triazine, 613.4 mg (5 mmol) zinc chloride to the sealed tube and grind with a mortar under infrared lamp Evenly, then put into the sealed tube. After the tube was sealed, it was placed in a muffle furnace at 600°C for 40 hours of reaction to obtain a polymer. After the reaction is complete, put the polymer in a 500ml flask, add 200ml of distilled water, ultrasonicate for 1h, then wash at 90°C for 12h under stirring, filter, then stir in 0.1M dilute hydrochloric acid for 12h, filter, wash with water until neutral, and then use Washing with dimethyl sulfoxide and acetone, the polymer is obtained after vacuum drying, and its synthetic route is as follows: Figure 10 Shown; its nitrogen adsorption-desorption curve and pore structure distribution are as follows Figure 11 and Figure 12 Shown; its carbon dioxide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Carbon dioxide adsorption capacity | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com