Preparation method of 1,5-naphthalene diisocyanate
A technology of naphthalene diisocyanate and acid-binding agent, which is applied in the field of preparation of 1,5-naphthalene diisocyanate, which can solve the problems of unconformity with atom economy, complex process flow, and highly toxic phosgene, and achieve redox catalytic performance Excellent, simple process, high reactivity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
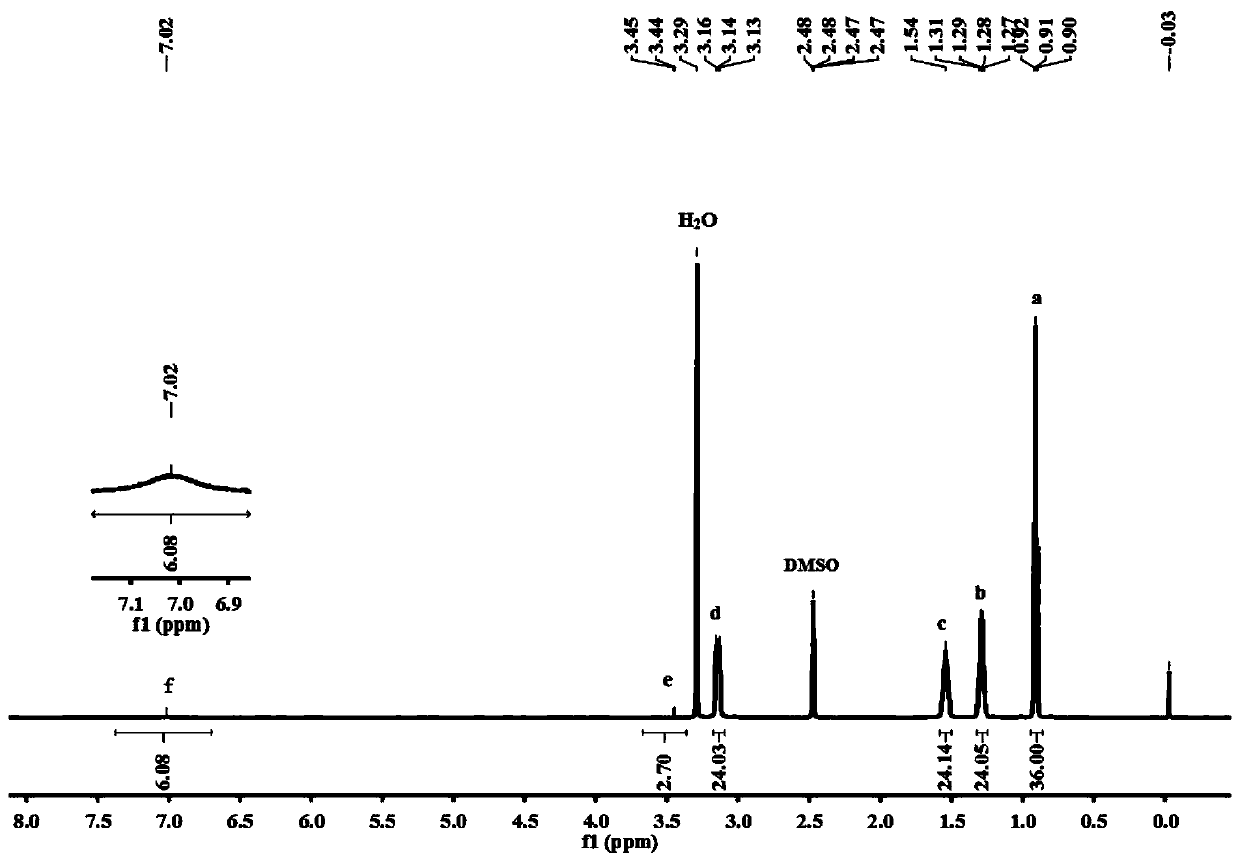
[0027] Add 0.0240g (0.02mmol) copper-centered polyoxometalates [NH 4 ] 4 [CuMo 6 o 18 (OH) 6 ]·7H 2 O(CuMo 6 ), 0.3164g (2mmol) 1,5-diaminonaphthalene, 6mL anhydrous acetonitrile solvent, 0.2164g (4mmol) phenylsilane, 0.1012g (1mmol) triethylamine, 0.3067g (2mmol) phosphorus oxychloride, and finally in An oxygen balloon filled with carbon dioxide was put over the reaction tube and reacted at 30°C for 12h. After the reaction, the above sample was taken to measure GC-MS. The test analysis showed that the conversion rate of the reaction substrate was greater than 93%, and the product selectivity was 89%. After separation and purification, a light yellow crystalline solid was obtained, which was confirmed by NMR to be the product 1,5-naphthalene diisocyanate.
Embodiment 2
[0029] Add 0.0240 g (0.02 mmol) of chromium-centered polyoxometalates [NH 4 ] 3 [CrMo 6 o 18 (OH) 6 ]·7H 2 O(CrMo 6 ), 0.3164g (2mmol) 1,5-diaminonaphthalene, 6mL anhydrous acetonitrile solvent, 0.2164g (4mmol) phenylsilane, 0.1012g (1mmol) triethylamine, 0.3067g (2mmol) phosphorus oxychloride, and finally in An oxygen balloon filled with carbon dioxide was put over the reaction tube and reacted at 30°C for 12h. After the reaction, the above sample was taken to measure GC-MS. It was found that the conversion rate of the reaction substrate was greater than 90%, and the selectivity of the product was 91%. After separation and purification, a light yellow crystalline solid was obtained, which was confirmed to be the product 1,5-naphthalene diisocyanate by NMR. .
Embodiment 3
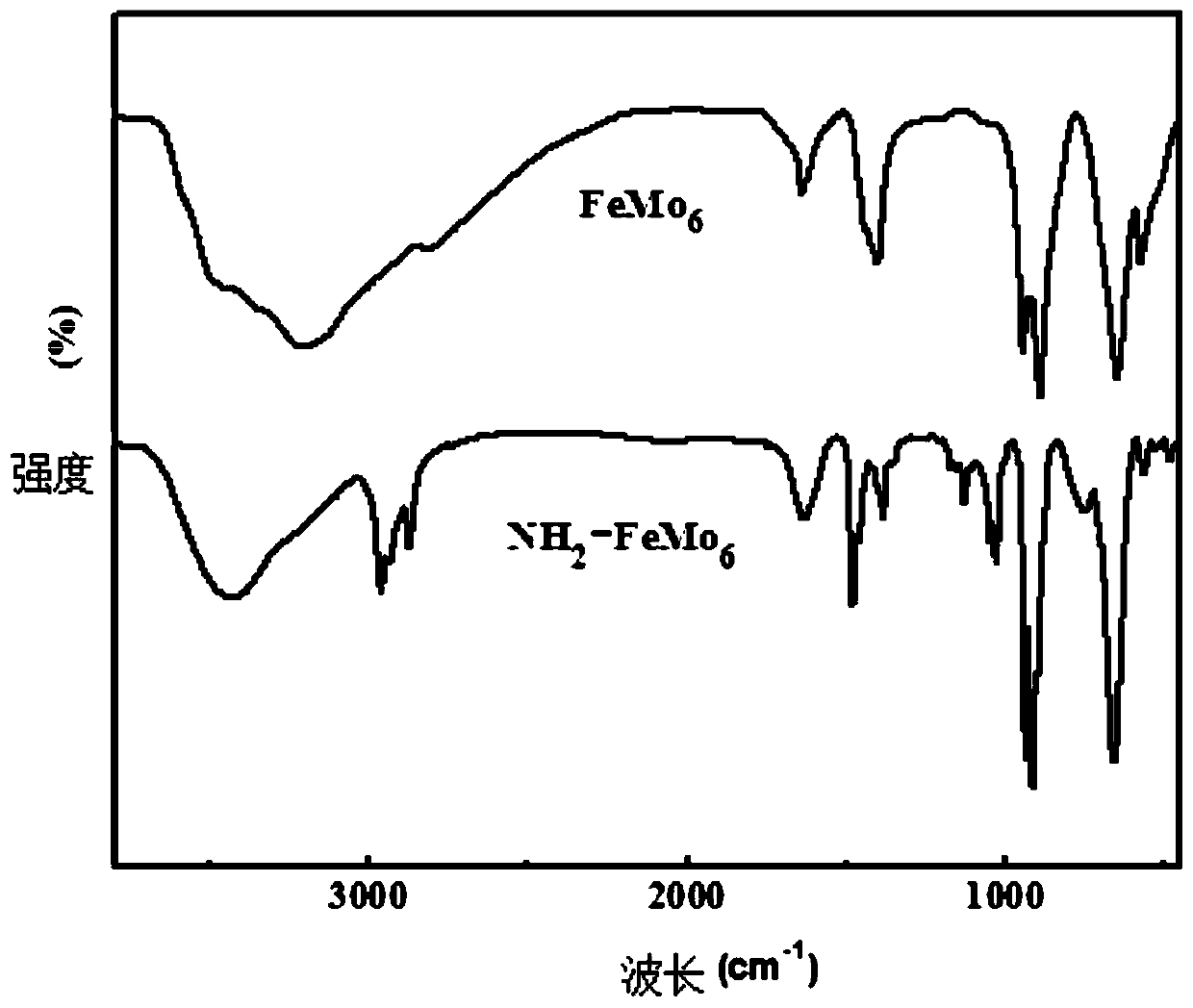
[0031] Add 0.0240 g (0.02 mmol) iron-centered polyoxometalate [NH 4 ] 3 [FeMo 6 o 18 (OH) 6 ]·7H 2 O(FeMo 6 ), 0.3164g (2mmol) 1,5-diaminonaphthalene, 6mL anhydrous acetonitrile solvent, 0.2164g (4mmol) phenylsilane, 0.1012g (1mmol) triethylamine, 0.3067g (2mmol) phosphorus oxychloride, and finally in An oxygen balloon filled with carbon dioxide was put over the reaction tube and reacted at 30°C for 12h. After the reaction, the above sample was taken to measure GC-MS. It was found that the conversion rate of the reaction substrate was greater than 96%, and the selectivity of the product was 93%. After separation and purification, a light yellow crystalline solid was obtained, which was confirmed to be the product 1,5-naphthalene diisocyanate by NMR. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com